Amphetamine medication (AMP) for ADHD
In the USA, amphetamine drugs are available as:1
- Mixture of D- and L-amphetamine isomers (racemic mixture)
- Mixed sulfates and saccharinates of D-L-amphetamine isomers (Adderall®)
- Pure D-amphetamine sulphate
- Dexamfetamine hemisulfate (Attentin®, Amfexa®)
- D-amphetamine as lisdexamfetamine in lysine-bound form (Vyvanse®, Vyvanse®, Tyvense®, generics)
- Racemic methamphetamine sulphate (Desoxyn®, USA)
In Germany, amphetamine drugs had to be prepared from raw substances by pharmacists for a long time.2 Since 2011, a D-amphetamine has been available in Germany as a finished drug and approved for the treatment of ADHD (Attentin), and in 2013 a D-amphetamine prodrug was approved for the treatment of children. Lisdexamfetamine contains D-amp in a lysine-bound form (Vyvanse). Since May 2019, Vyvanse Adult has been approved for the treatment of ADHD in adults (30, 50, 70 mg). In 2023, 20, 40 and 60 mg were also approved for adults. Since March 2024, Vyvanse and Vyvanse Adult have been combined to form the drug Vyvanse and are available in 20, 30, 40, 50, 60 and 70 mg in Germany.3 Since March 2024, Vyvanse has also been indicated as a first-line treatment for adults according to the Takeda prescribing information, but is still only indicated for children if MPH has been insufficiently effective.4 Ratiopharm’s prescribing information also does not mention the need for prior treatment with MPH.5
In Austria, Vyvanse can be prescribed if other medications are ineffective or show side effects. The doctor must justify this to the insurance company.
Amphetamine medication works slightly better in adults than methylphenidate6 and has slightly fewer side effects.
According to the current European consensus, amphetamines are the first choice of medication for ADHD in adults (before methylphenidate) and the second choice of medication in children and adolescents (after methylphenidate).78 The 2017 S3 guideline points out in the long version9 (page 72/198) that lisdexamfetamine can only be used in accordance with approval after prior treatment with MPH, without restricting this statement to children.
According to other sources, lisdexamfetamine has been approved for the first-line treatment of adults since 2019 and 2024 respectively.1011121314
The British NICE guideline (NICE, 2018), which, like the German guideline, is considered by experts to be based on the highest level of scientific evidence, explicitly recommends the use of LDX as a first-line therapy for the treatment of adults with ADHD.15
Lisdexamfetamine is approved in Germany for adults without age restriction and therefore also for seniors aged 60 and over. Nevertheless, there are no data on the safety and efficacy of lisdexamfetamine in people aged 60 and over. Methylphenidate and all other stimulants must be used off-label from the age of 60.16
Due to the responder/non-responder profile, which differs from MPH, amphetamine medications are particularly suitable for people with ADHD who do not respond to MPH, and clearly before the use of non-stimulants (e.g. noradrenergic medications or tricyclic antidepressants).17 A summary of several studies reports a 69% response rate to amphetamine medication and a 59% response rate to methylphenidate. 87 % of people with ADHD responded to one of the two types of drugs.18
Amphetamine medications are also suitable - unlike MPH - for the co-treatment of comorbid dysphoria or depression.1920
According to a Cochrane study, all amphetamine medications work equally well in adults, regardless of the specific form of medication.21 This distinguishes amphetamine medication from methylphenidate, where even a switch to another methylphenidate preparation shows considerable individual differences.
In practice, however, there are increasing reports that different lisdexamfetamine preparations can (but by no means have to) show considerable intraindividual differences. This also applies to people with ADHD who had not previously thought that there could be differences. Some people with ADHD reported that they were able to reliably reproduce differences in effect by switching between different preparations on a daily basis. There is no pharmacological explanation for this.
In studies on the effects of amphetamine, it must always be borne in mind that these
- usually use AMP in significantly higher doses than for ADHD medication
- generally use immediate release / not prolonged-acting AMP via prodrug
- frequently inject AMP, which again results in much faster metabolization
- these 3 factors multiply in their effect
There is no doubt that AMP in drug form has a different effect than AMP in drug form.
- 1. Active ingredients of amphetamine drugs
- 2. Amphetamines are similar to dopamine and noradrenaline
- 3. Amphetamine drugs work differently and in different parts of the brain than methylphenidate
- 3.1. Dopamine with amphetamine medication
- 3.1.1. Effect on DAT
- 3.1.2. Vesicular release
- 3.1.3. D2 autoreceptor activation
- 3.1.4. Increase in tyrosine hydroxylase
- 3.1.5. Increased DA firing / activation in dopaminergic brain regions
- 3.1.6. Reduced DA firing in the nucleus accumbens
- 3.1.7. Extracellular DA in the striatum increased more than by MPH
- 3.1.8. Synaptic DA binding reduced by AMP in the same way as by MPH
- 3.1.9. DA influence indirectly via effects on dopamine cells emanating from other brain regions
- 3.1.10. Downregulation of dopamine receptors?
- 3.2. Noradrenaline with amphetamine medication
- 3.3. Monoamine
- 3.4. Serotonin
- 3.5. Effect on the HPA axis
- 3.6. Inhibition of OCT2
- 3.7. Other effects on brain functions
- 3.8. Overview of AMP and neurotransmitters
- 3.1. Dopamine with amphetamine medication
- 4. Effect of amphetamine medication compared to MPH / atomoxetine
- 5. Effect on ADHD symptoms
- 6. Response rate (responding / non-responding)
- 7. No gender-specific differences in effectiveness
- 8. Calming effect at low doses, activating at high doses
- 9. Dosage of amphetamine medication or MPH
- 10. Effect profile (temporal) / duration of action
- 11. Areas of application of amphetamine drugs in relation to MPH
- 12. Side effects
- 13. Breakdown of amphetamine
- 14. Contraindications and interactions
- 15. Long-term effect: No habituation effects of amphetamine medication
- 16. Preparations
- 17. Taking amphetamine medication abroad
1. Active ingredients of amphetamine drugs
AMP has a chiral center with two enantiomers:22
- Levo-AMP (l-AMP)
- Noradrenaline release as strong as or stronger than d-AMP
- Dextro-AMP (d-AMP)
- higher dopamine release than l-AMP
Around 1976 it became known that a mixture of L-Amp and D-Amp worked better than D-Amp, which until then had been used alone23
The d-isomer is four times more effective at releasing dopamine than the l-isomer, while noradrenaline is released equally by both isomers or even slightly more by l-amphetamine23
The amphetamine mixed salt preparations available in the USA, which consist of equal parts racemic d,l-AMP sulfate, d,l-AMP aspartate monohydrate and two enantiomeric pure d-AMP salts (d-AMP sulfate and d-AMP saccharate), resulting in a ratio of 3:1 between d-AMP and l-AMP isomers and salts, a relatively greater release of noradrenaline than pure d-AMP, but still a greater release of dopamine than noradrenaline in absolute terms.
The following are relevant for the treatment of ADHD:
1.1. Dextroamphetamine (D-amphetamine)
Dextroamphetamine is also known as dexamphetamine or dextroamphetamine sulphate.
Dextroamphetamine is the dextrorotatory (D-)enantiomer of amphetamine, as opposed to the levorotatory levoamphetamine (see below).
D-amphetamine drugs have a 3 to 4 times stronger effect on the central nervous system than racemic amphetamine drugs, with a simultaneous lower sympathomimetic effect in the periphery, which is why D-amphetamine drugs are preferred for ADHD treatment.24
D-amphetamine is only more potent than L-amphetamine with regard to the dopamine transporters, while the effect on noradrenaline transporters is roughly the same.25
This opens up the possibility of emphasizing dopaminergic (dexamphetamine) or balanced dopaminergic and noradrenergic (levoamphetamine) medication.
D-amphetamine is more activating than MPH and is therefore preferably recommended for ADHD-I.26
It is also often more effective than MPH for parallel dysthymia / dysphoria / depression due to the noticeable serotonergic effect27.
1.1.1. Dextroamphetamine without lysine binding
Trade name: Attentin (Germany since the end of 2011), Dexamine (Switzerland: as magistral formulation), Dexedrine
Duration of action approx. 6 hours, so that it is usually necessary to take it twice a day.
Increased potential for abuse as no lysine binding.
Medice (2017): Attentin® - Guide for prescribing physicians
1.1.2. Dextroamphetamine from lisdexamfetamine (with lysine binding)
Lisdexamfetamine (LDX) is a prodrug of D-amphetamine that is bound to L-lysine to form a substance that is ineffective in itself. Lisdexamfetamine is therefore an active ingredient that is first converted in the body into the actual active substance, in this case D-amphetamine. This means that there is a very low risk of abuse.28 The subjective effect of intravenous intake is identical to that of oral intake, and the Cmax of d-amp is also identical.23 This massively reduces the risk of abuse. Nevertheless, the effect is linear and dose-dependent up to 250 mg. LDX therefore does not offer protection against overdose.29
Lisdexamfetamine (LDX) bound to lysine is rapidly absorbed from the small intestine into the bloodstream. This occurs by active transport, presumably by the peptide transporter 1 [PEPT1]. Enzymatic hydrolysis of the peptide bond to release d-amphetamine into the blood occurs in the lysate and cytosolic extract of human erythrocytes, but not in the membrane fraction. This conversion is strongly inhibited by a protease inhibitor cocktail, bestatin and ethylenediaminetetraacetic acid, suggesting an aminopeptidase as the cause of hydrolytic cleavage of the LDX peptide bond. Aminopeptidase B does not appear to be the cause30
Due to the necessary and slow conversion step from LDX to d-AMP, the effect occurs approx. 1 hour later than when taking d-AMP sulphate.29 Unlike LDX, the pharmacologically active d-AMP crosses the blood-brain barrier and enters the CNS, where it exerts its effect.22
Since the effect is quite uniform over the duration of action, the unpleasant rebound effects known from MPH (short-term increased restlessness at the end of the effect) are eliminated or are significantly weaker.
The effect corresponds to D-amphetamine. A conversion table from dexamphetamine to Vyvanse can be found at ADHSpedia.31 Further conversion tables are available from Kühle32 and for American preparations from Stutzman et al.33
Trade names:
- Vyvanse (EU, since the end of 2013, for children, 20, 30, 40, 50, 60, 70 mg)34
- Vyvanse Adult (EU, since 01.05.19, for adults, 30, 50, 70 mg)34. Since 2023, 20, 40 and 60 mg have also been approved in Germany.
- Vyvanse and Vyvanse adult were combined in 2023 to form a single medicine with a single approval. It was already an identical product. Since March 2024, Vyvanse has been available in 20, 30, 40, 50, 60 and 70 mg in Germany for children and adults.
- Vyvanse (USA) is available in doses of 10 mg to 70 mg35 Lisdexamfetamine is also approved for binge eating in the USA.36
- Tyvense (USA) is available in doses from 20 mg to 70 mg
- Teva-Lisdexamfetamine (Canada) is available in doses of 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg and 70 mg37
Generics:
- Since August 2024, lisdexamfetamine has been available in Germany as a generic (e.g. Lisdexamfetamine Ratiopharm), whereby 100-capsule packs are also available on the market.
Lisdexamfetamine has only been classified as a BtM in Germany since 2013.
Austria appears to be the only country in which Vyvanse is not classified as a narcotic (Austrian term: addictive drug), even as of 2023.38
The half-life of the D-enantiomer is39
- for children aged 6 to 12 years 9 hours
- 11 hours for adolescents aged 13 to 17 years
- for adults 10 hours
Due to its long-lasting effect, lisdexamfetamine is subject to steady state formation. Steady state appears to be reached on day 5.40 Consequences of this are that when dosing lisdexamfetamine (Vyvanse), dosage titrations should not be made below a weekly rhythm.
1.2. Levoamphetamine (L-amphetamine)
Levoamphetamine (L-amphetamine) is the purely levorotatory isomer of amphetamine.
L-amphetamine is less potent than D-amphetamine in terms of dopamine transporters, while the effect on noradrenaline transporters is roughly the same.25 This makes it slightly more noradrenergic than D-amphetamine, but still predominantly dopaminergic.41
L-amphetamine increases blood pressure and pulse rate more than D-amphetamine42
The half-life of the L-enantiomer is39
- for children aged 6 to 12 years 11 hours
- 13 to 14 hours for adolescents aged 13 to 17 years
- for adults 13 hours
We are not aware of any ready-to-use L-amphetamine medication approved in Europe. It would have to be produced on individual prescription in pharmacies.
Equivalents of lisdexamfetamine:
| Lisdexamfetamine | Dextroamphetamine | Dextroamphetamine sulfate |
|---|---|---|
| 10 mg | 2.95 mg | 4.02 mg |
| 20 mg | 5.90 mg | 8.04 mg |
| 30 mg | 8.84 mg | 12.05 mg |
| 40 mg | 11.79 mg | 16.07 mg |
| 50 mg | 14.74 mg | 20.09 mg |
| 60 mg | 17.69 mg | 24.11 mg |
| 70 mg | 20.64 mg | 28.13 mg |
The conversion rate from lisdexamfetamine to dextroamphetamine is 0.2948.43
The conversion rate of dexamphetamine to dexamphetamine sulfate can be estimated at 1.363.29
1.3. Mixed amphetamine salts / amphetamine derivatives
- Aderall (USA): 75 % dextroamphetamine and 25 % levoamphetamine
- Evekeo (USA): 50 % dextroamphetamine and 50 % levoamphetamine
Mixed amphetamine salts are a combination of different stimulants:44
D-amphetamine saccharate
D-amphetamine sulfate
D,L-amphetamine sulfate
D,L-amphetamine aspartate monohydrate
While D,L-amphetamine sulphate mixtures are the most commonly used ADHD medication in the USA, D,L-amphetemine mixtures are only available in a few pharmacies in Germany, which produce them themselves. The production is associated with a waiting time of several weeks. The cost of 180 capsules of 5 mg amphetamine sulphate each was quoted as €200.
1.4. Methamphetamine
- Desoxyn, USA
(1.5. Fenetyllin)
- Captagon (in Germany until 2003; in Belgium until 2010); no longer available today
2. Amphetamines are similar to dopamine and noradrenaline
Amphetamines are closely related in their chemical structure to the catecholamines dopamine and noradrenaline: this explains why they can bind to the receptors and transporters relevant for these. The great similarity between the monoamines also explains why monoamine transporters are relatively less selective, so that the noradrenaline transporter (at least in the PFC) reabsorbs more dopamine than noradrenaline.23
Source: Heal DJ, Smith SL, Gosden J, Nutt DJ (2013): Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol. 2013 Jun;27(6):479-96. doi: 10.1177/0269881113482532. PMID: 23539642; PMCID: PMC366619423, published under Creative Commons Attribution License
3. Amphetamine drugs work differently and in different parts of the brain than methylphenidate
Amphetamine drugs have a more complex mechanism of action than methylphenidate.
The description of the effects of amphetamine drugs is contradictory.
It is sometimes argued that amphetamine drugs merely inhibit dopamine reuptake and release dopamine and noradrenaline. More well-founded accounts from the USA (where amphetamine drugs are prescribed more frequently than in Europe and where there is therefore a more intensive debate about them) cite a reuptake inhibition of dopamine and noradrenaline transporters as the effect and no release of dopamine, noradrenaline or serotonin.
In the US, 52.9% of adolescents with ADHD receive MPH and 39.3% receive amphetamine medication as their first prescribed medication. Over the course of treatment, MPH is the primary prescribed medication for around 40% and 33% AMP is the primary prescribed medication.45
In principle, amphetamine drugs are said to have an intraneuronal effect, while methylphenidate and atomoxetine have an extraneuronal effect.46 As amphetamine drugs also address at least the dopamine transporter and the D2 autoreceptor, this is unlikely to be tenable.
AMP acts primarily in the striatum and further in the cortex and ventral tegmentum.47
The first computer models now exist that can seriously simulate the effect of ADHD drugs. A computer model for the simulation of type 1 diabetes has already been approved by the FDA as a replacement for preclinical animal studies48
A model comparing MPH and AMP in children and adults with ADHD takes into account the effect on 99 proteins involved in ADHD49
3.1. Dopamine with amphetamine medication
The dopamine increase caused by D-amphetamine in the PFC is much more pronounced and also much more dose-dependent than with MPH, and is therefore easier to control.46
AMP causes:
- extracellular dopamine levels increased 6-fold50
- tonic dopamine firing enhanced by AMP depleting vesicular stores and promoting non-exocytotic release through reverse transport51
- phasic dopamine firing: contradictory data
- amplified by upregulating vesicular dopamine release51
- Stimulants reduce the phasic release of dopamine50
- AMP promoted the release of dopamine from vesicles by reducing the affinity of vesicles for dopamine uptake (from K(m) 0.8 to K(m) 32). However, the amount of dopamine released per pulse was reduced by 82 % (according to another source by 25 to 50 %). The D2 antagonist sulpiride reduced the inhibition of release, i.e. promoted the release. This was reduced in D2-KO mice. In inhibited D2 autoreceptors, AMP increased the extracellular release of dopamine.52
- AMP reduces vesicular release5354 (this can affect both tonic and phasic release)
3.1.1. Effect on DAT
3.1.1.1. Dopamine (re)uptake inhibition via DAT and NET
Stimulants (MPH such as AMP inhibit dopamine reuptake55 and thus lead (in low doses) to a 6-fold increase in extracellular dopamine levels.50
The increased extracellular dopamine level acts on presynaptic dopamine D2 autoreceptors at the nerve ending. The D2 autoreceptor activation causes a 2- to 3-fold increase in impulse-associated (phasic) dopamine release. This increase is therefore relatively smaller than the increase in extracellular dopamine. The (relatively smaller) increase in phasic dopamine acts on the postsynaptic D2 dopamine receptors and causes reduced locomotor activity. Higher doses of stimulants increase extracellular dopamine more strongly and result in marked behavioral stimulation that cannot be overcome by phasic activation of inhibitory postsynaptic D2 receptors. High D-Amp doses cause supersaturation of extracellular postsynaptic D1 and D2 receptors, so that they exceed the inhibitory presynaptic effect of low D-AMP doses.50
- Amphetamine drugs block the dopamine and noradrenaline transporters in a different way to methylphenidate. While the reuptake inhibition of MPH is similar to that of antidepressants, amphetamine drugs act as a competitive inhibitor and pseudosubstrate on dopamine and noradrenaline transporters and bind to the same site where the monoamines bind to the transporter, thereby also inhibiting NE and DA reuptake.56
- D-amphetamine works
- Dextroamphetamine inhibits dopamine transporters with moderate efficacy (Ki 34-225 nM).58
- Amphetamines can also stabilize dopamine and norepinephrine transporters in channel configurations, reverse efflux through intracellular vesicular monoamine transporters, and cause internalization of dopamine transporters59
D-AMP drug doses cause a D-AMP plasma concentration of around 150 nM, which is sufficient to occupy a significant proportion of the dopamine transporters. This effect coincides with that of MPH.50 - D-amphetamine has approximately three times the affinity for noradrenaline transporters (NET) for reuptake inhibition and two and a half times the affinity for dopamine transporters (DAT) compared to racemic methylphenidate.46 Since there is barely any DAT present in PFC, but NETs are present, dopamine reuptake in PFC occurs primarily through the NET in noradrenergic cells.23
3.1.1.1.1. DAT inhibition via PKC
- AMP possibly inhibits DAT via PKC60
- Several protein kinases regulate DAT function6162
- AMP increases the activity of striatal particulate PKC via a calcium-dependent signaling pathway63
- PKC activation leads to phosphorylation in the N-terminal of the rat striatal DAT64
- PKC activation stimulates DAT-mediated dopamine release60
- PKC inhibitors and the downregulation of PKC60
- Inhibit efflux
- Leave dopamine uptake unchanged
3.1.1.2. Increased release of dopamine (DAT efflux)
The increased DAT efflux increases extracellular dopamine.
Amphetamine drugs release dopamine into the extracellular space 46 5755
Amphetamines therefore not only act as dopamine reuptake inhibitors, but also reverse the DAT function so that the DAT not only does not reabsorb dopamine, but also releases it from the cell (efflux).652366
This is newly synthesized dopamine. There is no doubt that this is not a depletion of dopamine reserves, as amphetamine drugs would otherwise have no lasting effect.
It is questionable whether this is dopamine that was previously stored in vesicles. There is no doubt that amphetamine drugs (characteristic of drugs: high dose, rapid application, rapid onset of action) release dopamine. It is questionable whether this is also the case with amphetamine drugs (characteristic: drug-like = low dose, slow release, long-lasting effect), and if so, to what extent this is the case.
3.1.1.2.1. Via VMAT2 at high doses
(Only) at a very high dosage as a drug, amphetamines also act on the vesicular monoamine transporter 2 (VMAT2) for dopamine and noradrenaline and then trigger an accumulating release of dopamine from the synaptic vesicles. The high amount of dopamine is then swept out into the synaptic cleft by a reversal of action of the dopamine transporters. This mechanism does not take effect at the usual dosage as an ADHD medication.25 In other words: Amphetamines can enter presynaptic monoamine vesicles and cause an efflux of neurotransmitters towards the synapse.67
An administration of 1 mg/kg AMP (injected) already caused a dopamine DAT efflux that was significantly higher at 10 mg/kg.68
3.1.1.2.2. By increasing intracellular Ca2+
AMP increases intracellular Ca2+, which supports phosphorylation of DAT at the N-terminus of the transporter. Phosphorylation (by CaMKII and possibly also by PKCβ) increases probability of DAT efflux from cytoplasmic DA.69
3.1.1.2.3. Increased DAT efflux via TAAR1
- AMP acts on DAT via TAAR1
Amphetamine enables the trace amine-associated receptor 1 (TAAR1) to phosphorylate the DAT transporter. This interrupts the reuptake of dopamine and the DAT is stimulated to release dopamine (efflux).67 - AMP also leads to increased intracellular accumulation of DAT70
3.1.2. Vesicular release
- AMP reduces vesicular release because as a lipophilic weak base and as a substrate for VMAT, AMP promotes the redistribution of dopamine from the synaptic vesicles into the cytosol by collapsing the vesicular pH gradient.53 As a result, AMP reduces the number of dopamine molecules released per vesicle.54
- Amphetamine initially reduces VMAT2, while prolonged administration increases it.71 MPH increases VMAT2 per se.7273
- AMP can inhibit vesicular release by indirectly activating D2 autoreceptors. The activation of D2 autoreceptors regulates potassium channels, which in turn regulate the probability of exocytic dopamine release.54
- A computer model determined74
- A maximum release of dopamine at 0.5-1.0 mg/kg AMP (lower at lower doses than at higher doses)
- Most of the dopamine released resulted from AMP-stimulated dopamine neosynthesis
- The dopamine produced was immediately converted into DOPAC, which is excreted extracellularly
- The dopamine was not stored in vesicles
- According to Stahl, AMP does not release dopamine, at least at low doses25
- AMP caused a gradual 10-fold increase in extracellular dopamine in the striatum over approximately 30 minutes in wild-type mice in vitro and in vivo, while simultaneously reducing the dopamine pool available for electrically stimulated release. If vesicular dopamine was previously released into the cytosol by reserpine, extracellular dopamine did not increase; however, AMP caused a rapid increase in dopamine within 5 minutes. In DAT-KO mice, extracellular dopamine did not increase, but at the same time electrically stimulable dopamine release was also reduced. DAT are therefore required for the dopamine-releasing effect of AMP, but not for the vesicle-emptying effect. Dopamine emptying of the vesicles is the rate-limiting step for the AMP effect on dopamine.75
- AMP (10 microm) promoted the release of dopamine from vesicles by reducing the affinity of vesicles for dopamine uptake (from K(m) 0.8 to K(m) 32 microm). However, the amount of dopamine released per pulse was reduced by 82 % (according to another source by 25 to 50 %). The D2 antagonist sulpiride reduced the inhibition of release, i.e. promoted the release. This was reduced in D2-KO mice.
In inhibited D2 autoreceptors, AMP increased the extracellular release of dopamine.52
- Emptying of the vesicular DA stores through a weak alkaline effect on the intravesicular pH gradient. The intravesicular pH gradient is required for the concentration of DA.
- Different effect on release-ready vesicles and reserve pool vesicles:51
- stimulus-dependent effect in the dorsal striatum
- stimulates vesicular dopamine release
- by a firing of short duration
- via vesicle pool ready for release
- Release reduced
- through a firing of long duration
- which accesses the reserve pool
- these opposing effects of vesicular dopamine release were associated with simultaneous increases in tonic and phasic dopamine responses
- stimulates vesicular dopamine release
- in the ventral striatum
- only increased vesicular release and increased phasic signals
- stimulus-dependent effect in the dorsal striatum
3.1.3. D2 autoreceptor activation
Basically, D-amphetamine activates D2 dopamine autoreceptors in the striatum.76
However, drug doses of D-AMP do not cause a significant reduction in dopamine release via activation of the D2 autoreceptors.7778
Since drugs such as levodopa or piribedil show no positive effect in ADHD, although they reduce the firing rate of the dopaminergic neurons of the substantia nigra pars compacta, it is doubtful whether the reduction of hyperactivity in ADHD by stimulants is based on presynaptic inhibition. Presumably, the reduction of hypermotor activity by stimulants in ADHD is rather based on an increase in dopamine release.77
3.1.4. Increase in tyrosine hydroxylase
Amphetamine drugs appear to have an activating effect on tyrosine hydroxylase in the dorsal striatum and nucleus accumbens, leading to increased L-dopa levels, but this does not appear to occur via a change in the phosphorylation of tyrosine hydroxylase.79
3.1.5. Increased DA firing / activation in dopaminergic brain regions
3.1.5.1. Increased DA firing in caudate nucleus / putamen (striatum)
High (well above drug dose) D-amphetamine administration (2.5 to 10 mg/kg in the rat into the abdominal cavity), leads to increased dopaminergic firing in the caudate nucleus and putamen and causes focused-repetitive (stereotypic) behavior.8081 The D2 antagonist haloperidol (2 mg/kg) terminates the excessive firing in the caudate nucleus and putamen and the reduced firing in the nucleus accumbens80
3.1.5.2. Increased DA firing in VTA and substantia nigra
D2 antagonists prevent increased firing in the substantia nigra and VTA (in vivo).82
3.1.5.3. Increased activation in the right orbitofrontal cortex, left middle frontal lobe, superior frontal lobe and precentral gyri
Improvement in ADHD symptoms with LDX was associated with significantly increased activation in a number of brain regions previously implicated in reinforcement processing under choice and feedback conditions (e.g., left caudate and putamen, right orbitofrontal cortex, left middle frontal lobe, superior frontal lobe, and precentral gyri).83
3.1.6. Reduced DA firing in the nucleus accumbens
In the nucleus accumbens, 7.5 mg/kg D-Amp led to a reduction in dopaminergic firing.80 The D2 antagonist haloperidol (2 mg/kg) terminated the excessive firing in the caudate nucleus and putamen and the reduced firing in the nucleus accumbens80
3.1.7. Extracellular DA in the striatum increased more than by MPH
In rats, amphetamine increased extracellular dopamine in the striatum by 1,400%, four times as much as MPH (+360%).84
3.1.8. Synaptic DA binding reduced by AMP in the same way as by MPH
In both rats and primates, amphetamine reduced the synaptic binding of dopamine in the striatum to the same extent as MPH (around 25% reduction).84
3.1.9. DA influence indirectly via effects on dopamine cells emanating from other brain regions
Amphetamine appears to influence the activity of dopamine cells indirectly via its effects on dopamine cells originating in other brain regions.85
Amphetamine can excite dopamine neurons by modulating glutamate neurotransmission. Amphetamine strongly inhibits inhibitory postsynaptic potentials in dopamine neurons mediated by the metabotropic glutamate receptor (mGluR), but has no effect on excitatory postsynaptic currents mediated by the ionotropic glutamate receptor. Amphetamine desensitizes mGluR-mediated hyperpolarization by:86
- DA release
- Activation of postsynaptic alpha1-adrenergic receptors
- Suppression of InsP3-induced calcium release from internal stores
By selectively suppressing the inhibitory component of glutamate-mediated transmission, amphetamine can promote burst firing of dopamine neurons and thus increase the phasic release of dopamine.
3.1.10. Downregulation of dopamine receptors?
Reports of immediate downregulation of dopamine receptors by administration of amphetamine are based on studies in which rats were given doses of amphetamine. This concerns the dosage level (5, 10, 15 mg/kg for 4 or 20 days twice daily) as well as the form (injection).87 Interestingly, a single dose of D-AMP even increased the number of receptors.8778
So far, we are not aware of any reports of downregulation when administered in the dose and form of medication.
Similarly, only studies with drug doses of amphetamines appear to change the dopamine receptor affinity or receptor status from high-affinity to low-affinity. Drug doses could alter the balance between receptor status towards low-affinity.78
More on receptor status at High-affinity and low-affinity receptor status In the article Dopamine effect on receptors
However, it is conceivable that amphetamine in drug doses does not cause a desensitization of the postsynaptic or extrasynaptic (the majority of dopamine receptors are located outside of synapses) D1 and D2 receptors directly, but via the detour of increasing the extracellular dopamine level. However, this hypothesis has not yet been experimentally proven.78 It is possible that this pathway leads to reduced psychomotor activity through amphetamine medication. In our view, however, this is contradicted by the fact that this effect already occurs with the first dose. On the other hand, this pathway could explain why many people with ADHD benefit from a slow and small-step titration of stimulants.
3.2. Noradrenaline with amphetamine medication
While D-Amp and L-Amp increase extracellular dopamine in the PFC and striatum in a dose-dependent manner, they increase extracellular noradrenaline only in the PFC.23
A: extracellular noradrenaline in the PFC, dose-dependent change due to D-Amp and L-AMP
B: extracellular dopamine in the striatum, dose-dependent change through D-Amp and L-AMP
Source: Heal DJ, Smith SL, Gosden J, Nutt DJ (2013): Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol. 2013 Jun;27(6):479-96. doi: 10.1177/0269881113482532. PMID: 23539642; PMCID: PMC366619423, published under Creative Commons Attribution License
3.2.1. Noradrenaline reuptake inhibition via NET
- Amphetamine drugs block the dopamine and noradrenaline transporters in a different way to methylphenidate. While the reuptake inhibition of MPH is similar to that of antidepressants, amphetamine drugs act as a competitive inhibitor and pseudosubstrate on dopamine and noradrenaline transporters and bind to the same site where the monoamines bind to the transporter, thereby also inhibiting NE and DA reuptake.5688
- Dextroamphetamine inhibits noradrenaline transporters with moderate efficacy (Ki 39-55 nM).58
- Amphetamines can also stabilize dopamine and norepinephrine transporters in channel configurations, reverse efflux through intracellular vesicular monoamine transporters, and cause internalization of dopamine transporters.59
- D-amphetamine has about a third of the reuptake inhibition on the noradrenaline transporter (NET) and dopamine transporter (DAT) as racemic methylphenidate.46
- Amphetamine (as well as ephedrine) also inhibits the intracellular noradrenaline transporter, which takes up noradrenaline from the nerve cell into the vesicles (the neurotransmitter stores)88
3.2.2. Noradrenaline release
- Whether amphetamine has a noradrenaline-releasing effect when administered as a drug is the subject of controversial debate, as is the case with dopamine. There are voices against25 as well as in favor.5557
- D-amphetamine secondarily increases the release of noradrenaline.76 This is always the case with dopaminergic drugs due to the conversion of dopamine (approx. 5 to 10 %) into noradrenaline.
- There is no doubt that amphetamine drugs do not lead to a chronic depletion of noradrenaline reserves in the sense of a deficiency state. It is empirically proven that amphetamine medications do not cause long-term habituation effects in ADHD
2.5 mg/kg AMP in mice:89
- stereotypical behavior (a sign of strongly increased extracellular dopamine); as strong as 20 mg/kg MPH
- extracellular dopamine increased
- extracellular noradrenaline increased
- extracellular serotonin increased
3.2.3. Reduction of noradrenaline metabolites only in responders
- In several independent studies, D-amphetamine drugs were found to decrease the urinary metabolite of norepinephrine, MHPG. The decrease of MPHG in urine is thought to be an important indicator of stimulant onset, indicating a lowering of norepinephrine levels by dextroamphetamine drugs.90](https://psycnet.apa.org/psycinfo/1982-21744-001)
- Furthermore, the reduction in noradrenaline metabolites only occurs in people with ADHD who respond positively to dexamphetamine (responders).91
- Even with the administration of methylphenidate, only the responders showed a significant decrease in MPHG in the urine, while MPHG in the urine of the non-responders did not decrease.92
The authors conclude that the level of noradrenaline in ADHD is reduced. - Furthermore, several studies with people with ADHD found that behavioral improvements were proportional to the reduced noradrenal metabolite levels (using D-amphetamine medication).93
In contrast to the reduction of metabolites in urine by D-amphetamine, the noradrenaline increase in PFC mediated by D-amphetamine is approximately as pronounced as that of MPH, but is significantly more dose-dependent and therefore more controllable.46
3.2.4. DA firing and DA bursting increased via noradrenaline α1 receptors
D-Amp (1 to 2 mg/kg) acts via alpha1-adrenoceptors94 (but not via alpha2- or beta-adrenoceptors) to increase dopaminergic firing and bursting in substantia nigra and VTA (in vivo). This adrenergic pathway is normally masked by the reduction in dopaminergic firing mediated by D2 autoreceptors and is visualized by D2 antagonists or by simultaneous administration of D1/D5 and D2/D3/D4 blockers. The selective norepinephrine uptake blocker nisoxetine did not increase the DA firing rate, but did increase DA bursts.8295
D-amphetamine appears to activate the noradrenaline α1-receptor in the PFC, as the α1-receptor antagonist prazosin completely neutralized the effect of D-amphetamine in the PFC. In contrast, D-amphetamine does not appear to target either the α2 receptor or the β receptor, as the effect of D-amphetamine persisted when the α2 or β receptors were blocked.96
D-amphetamine promotes the up-state of cortical neurons by activating97
- Central α1A-adrenoceptors
- D1 receptors
- D2 receptors
- But not by D1 or D2 receptors alone
In contrast, the dopamine/noradrenaline precursor L-DOPA did not promote the up state.
Arousal is associated with an increased up state, while slow-wave sleep, general anaesthesia and calm wakefulness are characterized by an oscillating change between up and down states. During arousal, the down states end and the up/down oscillation changes to a sustained up state.
The up/down oscillations appear to be relevant for memory consolidation, while the transition to a sustained up state is required for arousal and attention.97
3.3. Monoamine
3.3.1. Monoamine degradation inhibition via MAO
Amphetamine drugs act - albeit rather weakly23 - as MAO inhibitors,9847 unlike low-dose MPH. Whether high-dose MPH acts as an MAO inhibitor is unknown.46
MAO is an enzyme that breaks down dopamine and noradrenaline in the cell. MAO inhibitors thus increase the amount of dopamine and noradrenaline available in the cell. As dopamine and noradrenaline continue to be synthesized in the nerve cell, the noradrenaline and dopamine levels in the cell continue to rise. This leads to a reversal of the effect of the transporters (which actually return DA and NE from the synaptic cleft into the cell), so that they release NE and DA into the synaptic cleft, even without this being triggered by a nerve signal to be transmitted.98 This effect triggers peripheral hypertension and an increase in heart rate. As this mechanism of action occurs indirectly at the presynapse, ephedrine and amphetamine drugs are also called “indirect sympathomimetics”, while active ingredients that act directly at the postsynaptic receptors are called sympathomimetics.98
3.3.2. Monoamine release
Dextroamphetamine increases monoamine release from presynaptic terminals99, possibly via interaction with vesicular monoamine transporter 2 and reversal of plasma membrane monoamine transporters.58
3.4. Serotonin
3.4.1. Serotonin reuptake inhibition
Dextroamphetamine also inhibits serotonin transporters (Ki 1.4-3.8 μM) to a small extent.99
3.4.2. Serotonin release
Amphetamine drugs are said to release a small amount of serotonin.10024 Here, too, it is unclear whether this is really also the case when dosed at drug level, or whether this effect is only limited when dosed as drugs. In any case, Stahl does not report a serotonergic effect of amphetamine drugs.56
2.5 mg/kg AMP in mice:89
- stereotypical behavior (a sign of strongly increased extracellular dopamine); as strong as 20 mg/kg MPH
- extracellular dopamine increased
- extracellular noradrenaline increased
- extracellular serotonin increased
Serotonin release through amphetamine drugs
Amphetamine drugs (MDMA, MBDB) also increase the release of serotonin. It is assumed that amphetamine-induced serotonin release not only influences psychomotor activation, but also subjective well-being (and euphoria when taken as a drug).101 MDBD causes almost no dopamine release.
Hyperactivity induced by 5 mg or 10 mg/kg MDMA (= 10 to 20 times higher dosage than as a drug) could be prevented by prior administration of 2.5 and 10 mg/kg of the selective serotonin reuptake inhibitor fluoxetine. Fluoxetine had the same effect on the interactive effect of MDMA and P-chloroamphetamine.102 This suggests that MDMA causes hyperactivity by increasing serotonin via the serotonin transporter, which was blocked by fluoxetine as a serotonin reuptake inhibitor.
- There is evidence that an increased release of serotonin indirectly increases dopamine levels.102
- Other sources point to a serotonin-increasing effect of amphetamine salts due to inhibition of monoamine oxidase.27
- Amphetamine increases c-Fos expression in the mPFC, striatum and nucleus accumbens. A serotonin 1A receptor agonist reduced the c-Fos increase in the mPFC and striatum, but not in the nucleus accumbens.103
- MPH itself has an agonistic effect on the 5-HT1A receptor.47
3.5. Effect on the HPA axis
3.5.1. ACTH increased
Lisdexamfetamine and d-amphetamine significantly increased plasma ACTH levels in healthy subjects.104
3.5.2. Corticosteroids increased
- D-amphetamine drugs such as lisdexamfetamine (Vyvanse) increase cortisol levels, but not testosterone levels.104
- The following were increased
- Glucocorticoids (as with methylphenidate; the increase was even greater with the drugs MDMA or LSD)
- Cortisol
- Cortisone
- Corticosterone
- 11-Dehydrocorticosterone,
- 11-Deoxycortisol
- Glucocorticoids (as with methylphenidate; the increase was even greater with the drugs MDMA or LSD)
- The following remained unchanged
- Mineralocorticoids
- Aldosterone
- 11-Deoxycorticosterone
- Mineralocorticoids
The increase in the cortisol level causes a stronger addressing of the glucocorticoid receptor (GR) by cortisol. Cortisol causes the HPA axis to be switched off again via GR at the end of the stress response.
In ADHD-HI and ADHD-C (both with hyperactivity), due to the flattened endocrine stress response of the adrenal gland, it can be assumed that the GR are not sufficiently addressed to switch off the HPA axis again after a stress reaction. In addition, in ADHD-HI (unlike ADHD-I) there is often deficient GR function, which makes HPA axis deactivation even more difficult.
Find out more at Medication for ADHD at ⇒ Dexamethasone for ADHD. If the release of cortisol is now increased by AMP, this could improve the resilencing of the HPA axis in ADHD-HI. However, as AMP also works in ADHD-I, the primary mechanism of action is likely to be different.
3.5.3. Increased steroid hormones
Lisdexamfetamine and d-amphetamine significantly increased plasma levels in healthy subjects of, among others:104
- Androgens
- Dehydroepiandrosterone
- Dehydroepiandrosterone sulfate
- Androstenedione (Δ4-androstene-3,17-dione)
- Progesterone (only for men)
The androgen remained unchanged
- Testosterone
Since aggression correlates with an increased testosterone to cortisol ratio, amphetamine drugs have an anti-aggressive effect due to the relative increase in cortisol levels.
More on this at ⇒ Neurophysiological correlates of aggression
A study in adolescent rhesus monkeys found that both active ingredients increased testosterone levels, MPH even more than AMP, as a consequence of 12 months of AMP or MPH administration in drug doses.105 Another study in rhesus monkeys found reduced testosterone levels with MPH administration.106
A reduction in testosterone levels was observed in rodents following amphetamine administration.107108
3.6. Inhibition of OCT2
Basic information on uptake-2 transporters can be found at Dopamine degradation by organic cation transporters (OCT) In the article Dopamine reuptake, dopamine degradation
Organic cation transporter 2 (OCT2) is involved in the degradation of dopamine. OCTs take up dopamine and noradrenaline as well as serotonin and, to a somewhat greater extent, histamine in glial cells, where they are broken down by COMT. OCT2 and OCT3 are also located on (also dopaminergic) neurons.
While methylphenidate only binds to OCT1 (IC50: 0.36) and neither to OCT2, OCT3 nor PMAT109, d-amphetamine acts as a highly effective hOCT2 reuptake inhibitor (Ki: 10.5 mM) and moderately effective hOCT1 reuptake inhibitor (Ki: 202 mM), while it only interacted with hOCT3 from 100 μM (Ki: 460 mM) (hOCT: human OCT) 109110
d-Amphetamine binds approximately equally strongly to hOCT2 and hOCT3 and to these by an order of magnitude (factor 10) weaker than to DAT110
Binding of amphetamine to OCT may contribute to cellular and behavioral effects of amphetamine.110
OCT2 reuptake inhibitors have an antidepressant effect.111 In addition, even much lower doses of venlafaxine or reboxetine have an antidepressant effect in OCT2-KO mice than in wild-type mice112
We think it is worth considering whether this approach could also support the effect of dopamine reuptake inhibitors in ADHD.
These correlations could also explain why AMP, which also acts as an OCT2 inhibitor, has a better antidepressant effect than MPH, which only binds to OCT1.
3.7. Other effects on brain functions
- D-amphetamine increases metabolism in the right caudate nucleus and decreases it in the right Rolandic region and in the right anterior inferior frontal regions.113
- Neuroprotective effect in case of stroke or traumatic brain injury
- D-amphetamine (like L-dopa, which has no effect on ADHD, although it has a dopaminergic effect) is also suitable for restoring brain function after strokes, but only if appropriate training measures are taken at the same time.114 D-amphetamine increases dopamine, which has a neurotrophic effect (promotes neuroplasticity). Dopaminergic drugs such as (D-)amphetamine drugs or MPH can therefore also support appropriate training measures (e.g. neurofeedback, cognitive behavioral therapy) in ADHD by reducing the restrictions on learning ability.
- Low-dose methamphetamine given within 12 hours of a stroke or traumatic brain injury was neuroprotective and improved cognitive abilities and functional behavior.115
- There are similar reports with regard to MPH, although it also seems to depend on the rapid administration after the craniocerebral trauma116
- Methylphenidate and amphetamine drugs increase the power of alpha (in rats), while atomoxetine and guanfacine do not.117
- Lisdexamfetamine (Vyvanse) has the following effects118
- Increased acetylcholine levels in the cortex
- Increased histamine levels in the cortex and hippocampus (which escitalopram given in parallel only prevents in the hippocampus)
Amphetamine medication is therefore not just a substitute for methylphenidate, but has its own area of application.
3.8. Overview of AMP and neurotransmitters
3.8.1. Binding affinity of AMP, MPH, ATX to DAT / NET / SERT
The active ingredients methylphenidate (MPH), d-amphetamine (d-AMP), l-amphetamine (l-AMP) and atomoxetine (ATX) bind with different affinities to dopamine transporters (DAT), noradrenaline transporters (NET) and serotonin transporters (SERT). The binding causes an inhibition of the activity of the respective transporters.119
The values given in the following table by Easton et al. refer to values in the synaptosome as well as to the DAT in the striatum and the NET in the PFC.
| Binding affinity: stronger with smaller number (KD = Ki) | DAT | NET | SERT |
|---|---|---|---|
| MPH | 34 - 200119 , 34141 | 23841, 339119 | > 10,000119 |
| d-AMP (Vyvanse, Attentin) | 34 - 41119 , 206 (sulphate) 41 | ** 23.3 - 38.9**119 , 54.8 (sulphate)41 | 3,830 - 11,000119 |
| l-AMP | 138119 , 1435 (sulphate) 41 | ** 30.1**119 , 259 (sulphate)41 | 57,000119 |
| ATX | 1451 - 1600119 235541 | ** 2.6 - 5**119 , 20.641 | ** 48 - 77**119 |
| GBR-12909 | 40.241 | ||
| Desipramine | 4.941 |
3.8.2. Effect of AMP, MPH, ATX on dopamine / noradrenaline per brain region
The active ingredients methylphenidate (MPH), amphetamine (AMP) and atomoxetine (ATX) alter extracellular dopamine (DA) and noradrenaline (NE) to different degrees in different regions of the brain. Table based on Madras,119 modified.
| PFC | Striatum | Nucleus accumbens | |
|---|---|---|---|
| MPH | DA + NE (+) |
DA + NE +/- 0 |
DA + NE +/- 0 |
| AMP | DA + NE + |
DA + NE +/- 0 |
DA + NE +/- 0 |
| ATX | DA + NE + |
DA +/- 0 NE +/- 0 |
DA +/- 0 NE +/- 0 |
4. Effect of amphetamine medication compared to MPH / atomoxetine
In MPH nonresponders, lisdexamfetamine (EU: Vyvanse) and atomoxetine were compared in a randomized double-blind study with n = 200 subjects. Lisdexamfetamine was significantly more effective than atomoxetine in 2 of 6 categories and in the overall assessment.120
Lisdexamfetamine (EU: Vyvanse) also had a good effect on comorbid depression symptoms in a double-blind study.121 MPH is not known to have any positive effects on depression symptoms.
A 2-year study in children and adolescents (n = 314) showed a responder rate of between 70 and 77 % with good efficacy and manageable side effects.122
5. Effect on ADHD symptoms
In people with ADHD who respond positively to D-amphetamine medication as well as MPH, the effect of D-amphetamine medication is at least equal to MPH123, and in our experience in adults even significantly better.
For a comparison of the effectiveness of individual medications and forms of treatment, see ⇒ Effect size of different forms of treatment for ADHD.
According to the current European consensus, amphetamine medication is the first choice of medication for ADHD in adults (before methylphenidate) and the second choice of medication in children (after methylphenidate).78
Amphetamine medication should also always be tried if MPH does not work (non-responder).
5.1. ADHD-I (without hyperactivity)
MPH has a stronger activating and drive-enhancing effect on most people with ADHD than AMP medication. Contrary reports124 are not consistent with our experience.
Statements in the specialist literature that amphetamine medication is more suitable for people with ADHD-I than MPH, partly because people with ADHD-I are above-average AMP nonresponders,125 cannot be confirmed from our experience either
We know several persons with ADHD-HI who are significantly better helped by amphetamine medication than MPH and people with ADHD-I who cope better with MPH. We are not aware of any subtype-specific effect of amphetamine medication or methylphenidate. In our experience, amphetamine medication works just as well for ADHD-HI as for ADHD-I.
5.2. Attention control
People with ADHD have a reduced extrinsic and intrinsic motivational capacity. For example, they need higher rewards to be as motivated for something as non-affected people. However, once motivation is awakened in persons with ADHD, their attention and controllability can no longer be reliably distinguished from that of non-affected people. ⇒ Motivational shift towards own needs explains regulation problems
Attention correlates with a deactivation of the default mode network (DMN), among other things. Stimulants are able to align the attentional control of people with ADHD (or the motivational capacity, from which attention follows) with that of non-affected people, which is then also reflected in a normalization of DMN deactivation.126
More on the deviant function of the DMN in ADHD and its normalization by stimulants, including further sources at ⇒ DMN (Default Mode Network) In the article ⇒ Neurophysiological correlates of hyperactivity.
The cited references refer to the effect of methylphenidate. However, it can be assumed that the effect is achieved by stimulants in general.
People with ADHD report that MPH allows for greater focus, while amphetamine medications (Vyvanse) tend to create a more relaxed general alertness and have a slightly more pleasant effect overall.
5.3. Comorbid depression or dysthymia
Amphetamine medications probably also have a mild serotonergic effect and thus have a special area of application in comorbid dysthymia or depression, especially since serotonin reuptake inhibitors (SSRIs) can have adverse effects in ADHD (especially ADHD-I) (see there).
In forums, a number of people with ADHD report a significant antidepressant effect from amphetamine medication, which they are not familiar with from MPH.127 This is consistent with the experiences of users known to us.
As amphetamines can have a stronger drive-increasing effect than MPH, this can release an existing suicidal tendency that was not previously carried out due to the existing depression. Amphetamine medication should therefore be used with caution in cases of (even concealed) severe depression.
Attention: A supposed dysthymia (mild chronic depression) in people with ADHD must be clearly differentiated from the original ADHD symptom of dysphoria during inactivity.
Find out more at ⇒ Depression and dysphoria in ADHD In the section ⇒ Differential diagnosis of ADHD.
5.4. Comorbid anxiety disorders / depression
Comorbid anxiety disorders or depression can be exacerbated by stimulants, as anxiety and moods are regulated by the dopaminergic activity of the ventromedial PFC in conjunction with the limbic system.56
5.5. Comorbid sleep disorders
Amphetamine drugs have a very long duration of action (up to 13 hours). Taking it too late (less than 14 hours before going to bed) could therefore cause problems falling asleep. In contrast, some people with ADHD who take amphetamines report feeling pleasantly tired in the evening and that they no longer have problems falling asleep.
Studies show that amphetamine medications improve overall sleep quality in ADHD.128129
5.6. Impulsiveness
People with ADHD reported in forums that MPH worked better against impulsivity than Vyvanse (lisdexamfetamine).130
6. Response rate (responding / non-responding)
Response here means whether there is an effect on the ADHD symptoms. People with ADHD who do not respond sufficiently to a medication are called non-responders.
Non-responding does not mean having no effect, but merely that the effect remains below the level of symptom improvement specified in the respective study.
One study reported a responder rate of 80% (defined as an improvement of more than 30% in ADHD-RS-IV scores and greatly or very greatly improved CGI-I scores)131
A summary of several studies reports a 69% response rate to amphetamine medication and a 59% response rate to methylphenidate. 87 % of the people with ADHD responded to one of the two types of drug.18
A 2-year study of L-amphetamine medication in children and adolescents (n = 314) showed a responder rate of between 70 and 77% with good efficacy and manageable side effects.122
For MPH non-responders, it is therefore highly recommended to test a medication with amphetamine drugs (see 1.2.), and vice versa.
In carriers of the COMT Val-158-Met gene polymorphism, amphetamine increased the efficiency of the PFC in subjects with presumably low levels of dopamine in the PFC. In contrast, in carriers of the COMT Met-158-Met polymorphism, amphetamine had no effect on cortical efficiency at low to moderate working memory load and caused a deterioration at high working memory load. Individuals with the Met-158-Met polymorphism appear to be at increased risk for an adverse response to amphetamine.132
7. No gender-specific differences in effectiveness
Amphetamine drugs do not appear to show any gender-specific differences in effect.133
8. Calming effect at low doses, activating at high doses
D-amphetamine appears to have a biphasic action profile. Low doses of 0.5 to 1 mg/kg in rats (equivalent to about 0.2 to 0.6 mg/kg in humans) reduce (hyper)activity, while higher doses increase it.50
9. Dosage of amphetamine medication or MPH
About 66% of all persons with ADHD respond equally well to MPH as to amphetamine medication.
22% respond better to amphetamine drugs than to MPH.
11% respond better to MPH than to amphetamine drugs.134
Around 15% of people with ADHD respond best to the active ingredient D-amphetamine.135
According to this result, it would make more sense to first try therapy with amphetamine medication and only try MPH as a second option in the case of non-response, as people with ADHD respond somewhat better to amphetamine medication than to MPH.
Highly gifted people with ADHD (here: IQ > 120) are said to respond better to amphetamine medication than less gifted people with ADHD.136
An interesting study discusses the effectiveness of lisdexamfetamine.137
It is advisable to start with a very low dosage, which is then slowly increased. Even if the optimal dosage were known, an immediate optimal dosage would possibly lead to excessive demands.138 The symptoms of ADHD are caused by signal transmission problems between the brain nerves because the neurotransmitter level (dopamine, noradrenaline) is too low. An optimal neurotransmitter level corrects the signal transmission problems. If the neurotransmitter level is too high due to an overdose, signal transmission is just as impaired as if the level is too low.
This explains why low doses should be given at the beginning and then, with persistent persistence, higher doses should be given until a worsening of symptoms is observed.
As the number of dopamine transporters in adults is half that of 10-year-olds, it is advisable to start with a much lower dosage than in children.
10. Effect profile (temporal) / duration of action
In replicated studies on the duration of action of amphetamine drugs, children had a shorter half-life of around 7 hours, while adults had a longer half-life of around 10 to 12 hours139
The time course of the effect (effect profile) depends less on the active ingredients than on the specific composition of the medication.
Vyvanse has a very elongated effect profile without pronounced peaks, so that barely any flooding or rebound effects are noticeable. See: Graphic representation of the Vyvanse effect profile. However, the graph from Shire’s patent application refers to the plasma level in rats at an extremely high dose of 3 mg/kg.
Another graph shows the The progression of the active substance at 30 mg, 50 mg and 70 mg Vyvanseon page 20.
The extent to which the binding of D-amphetamine to lysine in lisdexamfetamine really leads to a flattened and prolonged concentration of amphetamine in the blood plasma remains to be seen. A single dose of 40 mg D-amphetamine or 100 mg lisdexamfetamine (above the medically appropriate doses) in healthy people showed no relevant differences in amphetamine blood plasma concentrations.140 Furthermore, the study data probably indicate a subjective impression of a gentler and longer effect of lisdexamfetamine on the part of the test subjects, although this is not reported by the authors. A further limitation of the study is that the test subjects were treated with a single dose and there was no dosing to the tested dosage. The authors themselves cite studies showing that amphetamine drugs require familiarization phases or show (initial) habituation effects. The results of the study are therefore primarily of pharmacological interest, but only of limited practical use.
Empirically, adults report quite unanimously of a gentler and prolonged effect of lisdexamfetamine. The majority cite 5 to 7 hours as the duration of action of a single dose. There is also a fairly unanimous report of a very slow onset of action, with 1 to 2 hours being mentioned in most cases.
An internal (and not representative) Survey at adhs-forum.adx.org on the duration of action of Vyvanse (n = 80) and another survey in a sub-reddit on Vyvanse (n = 467) yielded the following result (n = 547):
| Duration of action of a single dose of Vyvanse | % of participants |
|---|---|
| 5 hours and less | 40.8 % |
| 6 to 7 hours | 26.7 % |
| 8 to 9 hours | 15.4 % |
| 10 to 11 hours | 11 % |
| 12 hours and more | 6.2 % |
The surveys are not representative (no consideration of age, weight, dose level or gender), but clearly show that a duration of action of 13 or 14 hours, as stated by the manufacturer, is only exceptionally achieved in adults in practice.
A more detailed Survey on the single-dose duration of action of all ADHD medicationswhich also includes the aforementioned secondary factors, has been running since March 2023 and could show initial results in fall 2023.
Many people with ADHD (we know of dozens of cases from the forum) take 2 or 3 single doses of Vyvanse per day to achieve the required all-day coverage, even if this does not comply with the manufacturer’s instructions. The individually shortened duration of action could also be a consequence of a low dosage of often 30 mg or less per single dose, which was chosen when an overdose was perceived at a higher single dose during the phase of high D-AMP blood plasma levels. In almost no person with ADHD does the sum of the single doses exceed 70 mg / day.
The result of taking multiple smaller doses of Vyvanse on D-AMP blood plasma levels could (hypothetically) look like this:
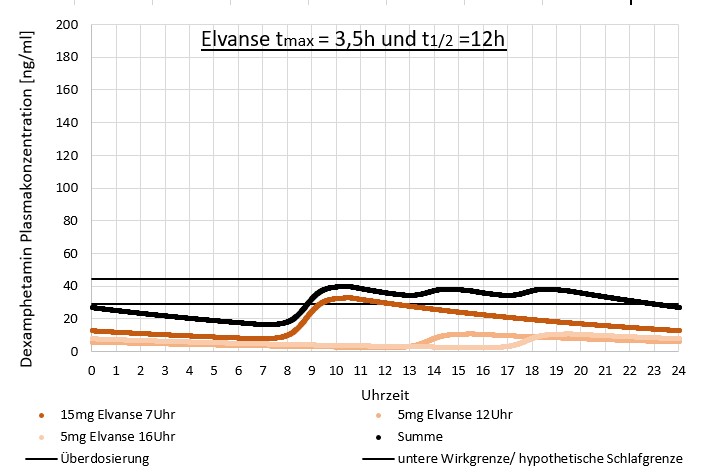
11. Areas of application of amphetamine drugs in relation to MPH
According to the current European consensus on the diagnosis and treatment of ADHD in adults, amphetamine medication is the first choice of medication for ADHD in adults (before methylphenidate) and the second choice of medication for children (after methylphenidate)78
In children who are MPH non-responders, i.e. who do not respond to MPH, the efficacy of amphetamine medication should be tested.
People with ADHD with pronounced dysphoria during inactivity or with comorbid depression benefit particularly from amphetamine medication.
In addition, people with ADHD who require stronger activation may be able to cope better with amphetamine medication.
Highly gifted people are said to respond better to amphetamine medication than to MPH.136
12. Side effects
12.1. No liver damage with normal medication dosage
High doses of amphetamines may be associated with liver damage and certain forms of clinically apparent liver damage. This is most commonly reported with methylenedioxymetamphetamine (MDMA: “ecstasy”).141
Amphetamine drugs, on the other hand, are dosed so low that this does not occur: the dose makes the poison. See also under ⇒ Amphetamine medication versus amphetamine as a drug.
12.2. AMP increases histamine
AMP increases histamine,142143 as do all other known ADHD medications:
- Atomoxetine
- Methylphenidate
- Modafinil
- Nicotine
- Caffeine
This is why people with histamine intolerance often have problems due to taking ADHD medication.
A person with ADHD with histamine intolerance reported that she could not tolerate AMP and sustained release MPH at all, but could tolerate immediate release MPH in small doses.
12.3. No increased cardiovascular risks
Several large studies found no increased risk of serious cardiovascular events such as stroke, heart attack or cardiac arrhythmia for amphetamine drugs.144145
A study over 14 years found a 4% increase in the risk of cardiovascular problems per year of taking stimulants (methylphenidate, amphetamines) and, to a lesser extent, the non-stimulant atomoxetine.146
According to a meta-analysis, daily use of amphetamine drugs had the following effects147
- systolic blood pressure increased by 1.93 mmHg (k = 56 RCT, n = 10,583)
- diastolic blood pressure increased by 1.84 mmHg (k = 56 RCT, n = 10,583)
- Heart rate increased by 3.71 beats per minute (k = 47 RCT, n = 10,075)
12.4. Individual cases of trichotillomania
Individual cases of trichotillomania (pulling out hair) have been reported.148 Trichotillomania is a specific form of impulse control disorder.
12.5. Erection, libido, reproduction
The package insert for Vyvanse mentions erectile dysfunction in 1 to 10 out of 100 men. However, the specialist literature or studies do not report any sexual impairments caused by amphetamine medication.
Reports from the ADxS-ADHD forum sometimes report erection problems with amphetamine medications, but barely with MPH.
Two male persons with ADHD reported a loss of sensitivity in the genital area after consuming red wine outside the active period of the regularly taken Vyvanse. In one of the persons with ADHD, low nicotine consumption outside the active period is another suspicious factor.
A single case report documents a reduction in testosterone and other sex hormones and a reduction in sperm count from an amphetamine medication, which was reversed by switching back to MPH.149
Amphetamine drugs also bind to alpha1-adrenoceptors (see above).
A blockade of alpha1-adrenoceptors leads to a delayed detumescence of the erectile tissue and thus to a reduced ability to ejaculate and orgasm, both in women and in men.150 Blockade is the opposite of binding. Dopamine agonists such as L-dopa or bromocriptine cause an increase in sexual desire and sexual activity.
Amphetamines (usually in drug use) can alter spermatogenesis and lead to oxidative stress and subsequent apoptosis in testicular tissue151
Amphetamine in drug doses (here: lisdexamfetamine) did not change the testosterone level.152
Amphetamine (in drug doses) is able to reduce testosterone production in rodents and increase the formation of cyclic AMP in the testes151
A single intravenous injection of amphetamine (administered as a drug) reduced hCG-stimulated testosterone release. The LH plasma level remained unchanged.
Amphetamine thus appears to have a direct and dose-dependent effect on Leydig cells, where it inhibits testosterone production by activating adenylate cyclase.107
A single intraperitoneal administration of methamphetamine initially lowered serum testosterone and increased it to a level above baseline after 48 hours.153
Chronic high methamphetamine administration reduced testosterone154 and increased GABA in the testes.155 GABA is involved in the proliferation of Leydig cells and testosterone production.
MDMA inhibits the hypothalamic-pituitary-gonadal axis in male rats. Acute and chronic MDMA administration resulted in decreased serum testosterone and GnRH mRNA expression. LH, progesterone and oestradiol remained unchanged. This indicates a reduced drive by hypothalamic GnRH neurons as a cause of inhibition of the hypothalamic-pituitary-gonadal axis.156
Subcutaneous MDMA administration for 12 weeks on three consecutive days/week (simulating human weekend use) did not alter the hormones of the hypothalamic-pituitary-gonadal axis.157
Methamphetamine can trigger apoptosis in testicular germ cells of mice158159 and reduce sperm count.160
Rats receiving 5 ml/kg methamphetamine intraperitoneally for 7 and 14 days (drug dose) showed significantly decreased spermatogonia, primary and secondary spermatocyte counts and spermatogenesis indices (tubule differentiation index, spermiogenesis index, repopulation index and mean testicular tubule diameter).161
MDMA is also capable of inducing histological changes in the testicles of rats and causing DNA damage to the sperm in a dose-dependent manner. However, the sperm count increased and the spermatid count decreased.157 MDMA increased the body temperature and the immunoreactivity of heat shock protein 70 (HSP70), which could activate apoptosis in the testicular tissue of the rat.162
A pilot study in men with sexual problems reported improvements in subjective sexual experience (reduced time to orgasm or increased frequency of orgasm) with 5 to 20 mg amphetamine salts (Adderall) 1 to 4 hours before sexual activity (up to 10 doses/month)163
In 5 individual cases, the resolution of SSRI-induced sexual dysfunction by small doses of dextroamphetamine or methylphenidate was reported.164 Further case studies report multiple erections (15-year-old), hypersexual behavior (8-year-old) due to OROS-MPH (Concerta)165 and priapism (14-year-old).166
One study reported a doubled rate of testosterone deficiency in adult persons with ADHD after 5 years of stimulant use (1.2%) compared to persons with ADHD without stimulant use (0.67%) or non-stimulant use (0.68%).167
12.6. Amphetamine drugs for the elderly
There are only a few studies on the effects and safety of amphetamine medication in older people.
One study found no increased risks for lisdexamfetamine in people between 55 and 84 years of age. There were no age-related trends in pulse or blood pressure changes and the safety profile of LDX was identical to that observed in younger adult study participants. The clearance of LDX decreased with age, so that a low dosage is reasonable or a prolonged effect can be expected.168
This is consistent with our known experience with stimulants in older people with ADHD. Nevertheless, particularly careful observation of the development of blood pressure is recommended.
12.7. Other
Common side effects of amphetamine mixed salts are:44
- Loss of appetite
- Mood swings
Rare serious side effects of amphetamine mixed salts are:44
- psychotic symptoms
- Seizures
- Risk of abuse
The drug MDMA (unlike amphetamine drugs) can damage nerve cells and attack the blood-brain barrier.169
12.8. Overdose
Symptoms of a (severe) overdose of amphetamines (in the sense of intoxication) include
- Agitation170
- Hyperactivity171
- Movement disorders170
- Tremor170
- Hyperthermia171
- Tachycardia (rapid heartbeat)171
- Tachypnea (increased respiratory rate)171
- Mydriasis (pupil enlargement)171170
- Trembling171
- Seizures171, in extreme cases up to epileptic forms170
- Hyperreflexia (excessive reflex response)170
- combative behavior170
- Confusion170
- Hallucinations170
- Delirium170
- Fear170
- Paranoia170
It is hardly surprising that doses of 11.3 mg / kg LDX trigger toxic effects in rats, as this is significantly higher than drug doses.172
13. Breakdown of amphetamine
13.1. Degradation of LDX to d-AMP
Lisdexamfetamine (Vyvanse) is converted to d-AMP in the blood cytosol of erythrocytes by an unknown amino acid (presumably an aminopeptidase)173174 by cleaving the covalent bond between d-amphetamine and L-lysine. Only d-AMP is pharmacologically active. d-AMP is degraded via CYP2D6.
96% of LDX is excreted in the urine, of which35
- 42 % of the dose as AMP
- 25 % as hippuric acid
- 2 % as intact LDX.
In contrast to AMP, LDX is less sensitive to changes in urine pH.
The half-life of LDX is typically less than 1 hour.
13.2. Degradation of D-AMP and L-AMP
D-AMP is metabolized faster than l-AMP, so that the exposure of d-AMP lasts 9-11 hours and of l-AMP 11-14 hours.
Taking it together with a high-fat meal can extend the half-life of d-AMP by one hour.
Two online surveys of a total of around 550 people with ADHD who take Vyvanse showed that around 40% have a duration of action of 5 hours or less and two thirds have a single-dose duration of action of 7 hours or less. More on this under Effect and duration of action of ADHD medication
Amphetamine is oxidized to39174
- 4-Hydroxyamphetamine (active metabolite)
- Degradation to 4-hydroxy-norephedrine
- Alpha-hydroxyamphetamine
- Dismantling via 2YP2D6
- Deamination to phenylacetone
- Glucuronidation to benzoic acid
- Degradation to hippuric acid (glycine conjugate)
- Norephedrine (phenylpropanolamine) (active metabolite)
- Degradation to 4-hydroxy-norephedrine
13.2.1. Primary degradation pathway: renal, acid-dependent
AMP is primarily excreted via the kidneys.
Since AMP is slightly basic (pKA = 9.9), AMP excretion is highly dependent on urine pH and flow rate, with recovery of AMP in urine ranging from 1% to 75% and the remainder being metabolized hepatically:35
- normal urine pH values
- 30 to 40 % of the AMP dose is largely excreted as unchanged parent compound
- 50% of the dose is excreted as alpha-hydroxyamphetamine or its downstream inactive metabolite, hippuric acid.
- acidic urine (pH <6.0)
- accelerated AMP excretion, up to 50 % of d-AMP is excreted unchanged renally. Weaker / shorter efficacy.
- alkaline urine (pH >7.5)
- delayed AMP excretion, only 3 % of d-AMP is excreted unchanged. Stronger / longer effectiveness.
The half-life of AMP should increase by 7 hours per unit of pH increase. Acidifying or alkalizing agents can therefore significantly alter the effect of AMP.
More on this under Acid balance and amphetamine medication In the article Strength and duration of action of ADHD drugs.
13.2.2. Secondary degradation pathway: CYP2D6, CYP1A2 and CYP3A4
Amphetamine is broken down by CYP2D6, CYP1A2 and CYP3A4.39
The CYP2D6 genotype influences the efficacy of amphetamine medications in ADHD, although it is unclear to what extent.
CYP2D6 weak metabolizers were 3.67 times more likely to show symptom improvement than intermediate CYP2D6 metabolizers (after correction for phenotype conversion and adjustment for gender, age, dose, duration, and treatment adherence). Self-reported adverse events were not influenced by CYP2D6 metabolizer phenotype.175 Poor metabolizers are likely to require lower AMP doses and ultra-rapid metabolizers are likely to require higher AMP doses. However, the effects of CYP2D6 polymorphisms on AMP metabolism are still unclear.35
Although CYP2D6 is involved in the degradation of d-AMP, it is not the dominant degradation pathway.176 For methamphetamine, CYP2D6 was named as the main degradation pathway, whereas MDMA is not oxidized by CYP2D6.177 Amphetamine itself appears to be metabolized to a much lesser extent by CYP2D6 than some amphetamine analogues.178179 In vivo, CYP2D6 appears to be less involved in the degradation of amphetamines than in vitro180
Regardless of this, amphetamine is a strong CYP2D6 inhibitor.179
The CYP2D6 gene is highly polymorphic. In Central Europe, the following alleles are particularly relevant181
- CYP2D6*3
- CYP2D6*4
- CYP2D6*5
- CYP2D6*6
- CYP2D6*9
- CYP2D6*41
Based on the experience with the influence of CYP2D6 on the effect of other drugs (CYP2D6 is responsible for the metabolization of 20 - 30 % of all drugs), the different CYP2D6 gene variants lead to different types of metabolization181
- Slow metabolizers - approx. 7 %
- particularly slow dosing is important
- particularly low dosage helpful
- moderately fast metabolizers - approx. 40 %
- Fast metabolizers - approx. 46 %
- Ultra-fast metabolizers - approx. 7 %
- CYP2D6*XN allele
- increased enzyme activity
- is associated with therapy resistance (non-responders)
- increased dose can be helpful
More on this under –> CYP2D6 metabolizing enzyme
In the ADxS forum, several people with ADHD reported a significant prolongation of the duration of action of a single dose of amphetamine medication by berberine, which is metabolized by CYP2D6 and CYP3A4, and is thus a competitor for these enzymes. Bupropion also prolongs the effect of amphetamine drugs. Bupropion is a strong CYP2D6 inhibitor and is not affected by CYP3A4.
14. Contraindications and interactions
As with every drug described here, there are also contraindications for amphetamine drugs.
It should not be taken without prior medical consultation.
- Pregnancy / breastfeeding
- No disadvantages for the child if the mother continued to take D-Amp during pregnancy. In contrast, there was an increased risk of abortion if D-Amp intake was discontinued during pregnancy. There were advantages if no D-Amp was taken before and during pregnancy. Early discontinuation could therefore be helpful if you wish to have children.182
- A cohort study found no increased risk of ADHD or other neuronal developmental disorders from MPH or AMP use during pregnancy.183
- Risk of neuronal developmental disorders for the offspring unchanged when taking ATX during pregnancy (Swedish cohort study, n = 861,650 children of n = 572,731 mothers from 2008-2017)184
- Weight of newborns
- Unchanged, of mothers with ADHD who took amphetamine medication during pregnancy.185 This is consistent with results from a large cohort study of MPH use during pregnancy.186
- a slight reduction in birth weight and a slight increase in the risks of pre-eclampsia, placental abruption or premature birth when taking stimulants (AMP or MPH) during pregnancy, although this was so small that the authors did not recommend discontinuing stimulant use during pregnancy.187 Atomoxetine did not show these slight increases in risk.
- Miscarriages
- Doubled risk of miscarriage when taking stimulants during pregnancy.188
- Malformations
For lisdexamfetamine:191
- Hypersensitivity to the active ingredient
- Monoamine oxidase inhibitors (MAO inhibitors) at the same time or 14 days before use
- Risk: hypertensive crisis
- Hyperthyroidism / thyrotoxicosis
- Excitation states
- Symptomatic cardiovascular disease
- Advanced arteriosclerosis
- Moderate to severe hypertension
- Glaucoma
- Serotonin reuptake inhibitors
- The risk of serotonin syndrome should be taken into account when administering SSRIs and amphetamine medication at the same time.27
According to a very large study, the risk of developing psychosis is lower for people with ADHD taking MPH (0.10%) than for those treated with amphetamine medication (0.21%).192 While people with ADHD treated with stimulants have 2.4 cases of psychosis per 1000 person-years, the figure for the population as a whole is 0.214%.193 The studies do not allow any conclusion to be drawn as to whether the increased prevalence of psychosis is attributable to ADHD or stimulants.
The studies do not allow any conclusion to be drawn as to whether the increase in the prevalence of psychosis is due to the existence of ADHD or to the administration of stimulants.
Another study found an 8-fold risk for people with ADHD when taking ADHD medication (0.34% vs. 0.048%).194 As this does not compare people with ADHD with and without medication, but people with ADHD with medication against the population as a whole, it is not possible to draw any reliable conclusions about the contribution of ADHD medication. The absolute risk of first-time psychosis or mania when starting medication was drug-dependent:
- Atomoxetine: 0.60 % (highest)
- Amphetamine drugs: 0.33 %
- Methylphenidate: 0.19 %
14.1. Shortened half-life (reduced effect)
Attenuation of the effects of dexamphetamine:195
- Adrenoreceptor blockers (beta blockers)
- e.g:
- Propranolol
- e.g:
- Lithium
- Phenothiazines
- Haloperidol
- Substances that lower the pH value in the gastrointestinal tract
- e.g:
- Guanethidine
- Reserpine
- Glutamic acid
- Hydrochloric acid
- Ascorbic acid
- Fruit juice
- cause reduced absorption of dexamphetamine
- e.g:
- Substances that acidify urine (ammonium chloride, sodium dihydrogen phosphate, etc.)
- increase ionized excretion products of dexamphetamine in urine, resulting in increased renal excretion
- Acidification of the urine (reduced pH value)191
e.g. through- Ascorbic acid
- Thiazide diuretics
- High protein diet
- Diabetes mellitus
Caution: Foods that taste acidic often have an alkalizing effect in the body beyond the digestive tract.
Example: Lemon juice has a pH value of 2.4 and therefore has an acidic effect on the mouth and stomach. After digestion, however, only an alkaline residue remains in the rest of the body, which increases the pH value.
The effect of food after digestion on the acid load of the kidneys due to minerals and protein is indicated by the PRAL value (potential renal acid load). This value is not suitable for assessing the acid load of the mouth and stomach (as is relevant for heartburn).
The higher the PRAL value, the more acidic the effect on the kidneys and the rest of the body after the digestive organs.
Urine pH has been shown to be a good PRAL marker. An alkaline urine pH value correlates with a diet with a negative PRAL value, while urine pH values below 6.0 correlate with an acidifying diet.
A distinction must be made between plant and animal proteins. After 7 days of a vegetarian diet, the pH urine value increases and the PRAL value decreases, as does 2 or 3 days of a vegetarian diet per week.196 A vegetarian diet thus correlates with a prolonged amphetamine drug effect.
Foods with a high oxalate content can increase acid formation.197
One study gives the following calculation method:198 PRAL (mEq/d) = 0.49 x protein (g/d) + 0.037 x phosphorus (mg/d) - 0.021 x potassium (mg/d) - 0.026 x magnesium (mg/d) - 0.013 x calcium (mg/d).
In other words, foods with a strongly negative PRAL value cause alkaline (less acidic) urine and thus promote a prolonged effect of amphetamine drugs. Foods with a high PRAL value cause acidic urine and thus promote a shortened effect of amphetamine drugs. According to this model, hard cheese is suitable for shortening the effects of amphetamine drugs, while raisins could prolong them.
More on this under Acid balance and amphetamine medication In the article Effect and duration of action of ADHD medication
14.2. Prolonged half-life (increased effect)
- With alkalized urine (increased pH value)191
e.g. through- Sodium hydrogen carbonate (baking powder, soda)
- Diet with a high fruit / vegetable content
- Urinary tract infections
- Vomiting
- Clonidine195
Enhanced effect of dexamphetamine due to:195
- Disulfiram
- Substances that increase the pH value in the gastrointestinal tract increase dexamphetamine uptake
- e.g:
- Sodium bicarbonate (baking powder)
- e.g:
- Substances that increase urine pH increase non-ionized excretion products in urine, which decreases renal excretion and thus increases blood levels of dexamphetamine
- e.g:
- Acetazolamide
- some thiazides
- e.g:
There is evidence that reduced expression of the CACNA1C gene can lead to a prolonged effect of dopamine reuptake inhibitors.199 Conversely, increased CACNA1C expression may lead to a shortened effect.
14.3. Delayed effect
Lisdexamfetamine (Vyvanse) has a maximum blood level that is delayed by one hour with high-fat meals (4.7 hours instead of 3.8 hours after ingestion).200 However, other parameters, such as the duration of action, do not change.
14.4. Amplifying effect on amphetamines
14.4.1. Alcohol increases amphetamine levels
Alcohol can increase amphetamine levels.201
14.4.2. CYP2D6 inhibitors increase amphetamine levels
As amphetamine is broken down by CYP2D6, drugs that are also broken down by CYP2D6 can slow down the breakdown of amphetamine as well as their own breakdown, as competition for the CYP2D6 enzyme arises.
CYP2D6 inhibitors can increase amphetamine levels, making a dose reduction necessary. After discontinuation of CYP2D6 inhibitors, an increase in the dose of amphetamine medication may be necessary.201
CYP2D6 inducers can accelerate degradation and thus reduce the effect.
See under CYP2D6 metabolizing enzyme
14.5. Attenuating effect on amphetamines
Have an attenuating effect on amphetamine:202
- Chlorpromazine
- Haloperidol
- Lithium carbonate
A single person with ADHD reported a loss of efficacy of Vyvanse due to Dienogest 2 mg (Zafrilla) in endometriosis, while the effect of Attentin remained unchanged.
14.6. Few interactions of AMP with other medications
In contrast to the above-mentioned interactions of amphetamine drugs with other drugs, barely any interactions of amphetamine drugs with other drugs are known.201
Amphetamine is said to have a slight inhibitory effect on the cytochromes
- CYP2D6
- CYP1A2
- CYP3A4.
The clinical relevance is classified as low.202
Attenuating effect on202
- Antihypertensives such as guanethidine
Reinforcing on202
- Analgesic effect of opioids
14.7. AMP during pregnancy
A small prospective study of n = 13 children whose mothers received amphetamine medication while breastfeeding found no disadvantages for the children.203
15. Long-term effect: No habituation effects of amphetamine medication
A meta-analysis of 87 randomized placebo-controlled double-blind studies found no evidence of a decrease in the effect of methylphenidate, amphetamine drugs, atomoxetine or α2 antagonists with prolonged use.204
16. Preparations
Amphetamine medications are available as various preparations.
In the USA, amphetamine drugs are available as:1
- Mixture of D- and L-amphetamine isomers (racemic mixture)
- Mixed sulfates and saccharinates of D-L-amphetamine isomers (Adderall)
- Pure D-amphetamine sulphate
- Dexamfetamine hemisulfate
- United Kingdom: Amfexa®
- Germany, Denmark, Finland, Iceland, Luxembourg, Norway, Sweden: Attentin®
- Approved for children and young people aged 6 to 17 years
- Doses: 5 mg tablets, 10 mg break-in tablets
- Dosage: 5-10 mg/day orally, with an increase of 5 mg per week, up to a maximum dose of 20 mg/day (up to 40 mg for older children in exceptional cases)205
- Dexamfetamine hemisulfate
- D-amphetamine as lisdexamfetamine in lysine-bound form (Vyvanse, Tyvense)
- Racemic methamphetamine sulphate (Desoxyn)
Vyvanse, Tyvense
Vyvanse / Tyvense contains lisdexamfetamine. Lisdexamfetamine is dextroamphetamine bound to lysine. The lysine binding causes a very slow and even release of dextroamphetamine in the blood and thus a prolonged effect.
Available in the USA:
Capsules: 10, 20, 30, 40, 50, 60, 70 mg
Chewable tablets: 10, 20, 30, 40, 50, 60 mg
17. Taking amphetamine medication abroad
See under Taking stimulants abroad
Edel, Vollmoeller (2006): Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung bei Erwachsenen, Springer, Seite 57, Stand 2006 ↥ ↥
so noch Edel, Vollmoeller (2006): Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung bei Erwachsenen, Springer, Seite 57 ↥
Takeda März 2024: Fachinformation Elvanse 20 mg/30 mg/40 mg/50 mg/60 mg/70 mg, unter 4.1. ↥
Ratiopharm (Juni 2024): Lisdexamfetamin-ratiopharm Erwachsene Hartkapseln, Version 5 ↥
Rösler, Retz (2020): Medikamentöse Therapie der ADHS bei Erwachsenen; Psychiatrie up2date 2020; 14: 59–75 ↥
Kooij, Bijlenga, Salerno, Jaeschke, Bitter, Balázs, Thome, Dom, Kasper, Filipe, Stes, Mohr, Leppämäki, Brugué, Bobes, Mccarthy, Richarte, Philipsen, Pehlivanidis, Niemela, Styr, Semerci, Bolea-Alamanac, Edvinsson, Baeyens, Wynchank, Sobanski, Philipsen, McNicholas, Caci, Mihailescu, Manor, Dobrescu, Krause, Fayyad, Ramos-Quiroga, Foeken, Rad, Adamou, Ohlmeier, Fitzgerald, Gill, Lensing, Mukaddes, Brudkiewicz, Gustafsson, Tania, Oswald, Carpentier, De Rossi, Delorme, Simoska, Pallanti, Young, Bejerot, Lehtonen, Kustow, Müller-Sedgwick, Hirvikoski, Pironti, Ginsberg, Félegeházy, Garcia-Portilla, Asherson (2018): Updated European Consensus Statement on diagnosis and treatment of adult ADHD, European Psychiatrie, European Psychiatry 56 (2019) 14–34, http://dx.doi.org/10.1016/j.eurpsy.2018.11.001, Seite 22, 7.4.1. ↥ ↥ ↥
Cortese, Adamo, Del Giovane, Mohr-Jensen, Hayes, Carucci, Atkinson, Tessari, Banaschewski, Coghill, Hollis, Simonoff, Zuddas, Barbui, Purgato, Steinhausen, Shokraneh, Xia, Cipriani (2018): Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018 Sep;5(9):727-738. doi: 10.1016/S2215-0366(18)30269-4. ↥ ↥ ↥
Banaschewski T (Leitlinienkoordinator), Hohmann, Millenet et al (2018): Langfassung der interdisziplinären evidenz- und konsensbasierten (S3) Leitlinie „Aufmerksamkeitsdefizit- / Hyperaktivitätsstörung (ADHS) im Kindes-, Jugend- und Erwachsenenalter“ AWMF-Registernummer 028-045 ↥
Döpfner, Universitätsklinikum Köln, https://www.adhs.info/fuer-erwachsene/therapie-und-andere-hilfen/, Ansicht 03.10.25 ↥
Lisdexamfetamin – jetzt auch für Erwachsene zugelassen – Kompendium psychiatrische Pharmakotherapie, Springer. ↥
Pharmazeutische Zeitung: Arzneistoffe: Lisdexamfetamin|Elvanse®|71|2013 ↥
Kölle, Mackert, Philipsen (2021): Pharmakotherapie der ADHS im Erwachsenenalter; Psychopharmakotherapie, Abruf 03.10.2025 ↥
Winkler (2024): Elvanse für Alle: Eine Neuerung in der ADHS-Behandlung, ADHSSpektrum.com, Abruf 03.10.2025 ↥
Zusammenfassende Dokumentation über eine Änderung der Arzneimittel-Richtlinie (AM-RL): Anlage XII – Nutzenbewertung von Arzneimitteln mit neuen Wirkstoffen nach § 35a SGB V Lisdexamfetamindimesilat (17. Oktober 2019) - ohne Stellungnahme GBA ↥
Hohmann-Jeddi (2024) Was bei Erwachsenen anders ist; Interviewaussage Retz; Pharmazeutische Zeitung. Ansicht ß3.10.2025 ↥
Frölich, Banaschewski, Spanagel, Döpfner, Lehmkuhl (2012): Die medikamentöse Behandlung der Aufmerksamkeitsdefizit-Hyperaktivitätsstörung im Kindes- und Jugendalter mit Amphetaminpräparaten; Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie (2012), 40, pp. 287-300. https://doi.org/10.1024/1422-4917/a000185 ↥
Arnold: Journal of Attention Disorders Vol. 3(4):200-211 (2000) Methylphenidate vs. amphetamine: Comparative review, n = 174 ↥ ↥
Krause, Krause (2014): ADHS im Erwachsenenalter, Schattauer. Fallbeispiel S. 155 ↥
Madhoo, Keefe, Roth, Sambunaris, Wu, Trivedi, Anderson, Lasser (2014): Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder; Neuropsychopharmacology. 2014 May;39(6):1388-98. doi: 10.1038/npp.2013.334. n = 143 Erwachsene ↥
Castells X1, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas (2011): Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults; Cochrane Database Syst Rev. 2011 Jun 15;(6):CD007813. doi: 10.1002/14651858.CD007813.pub2. n = 1071 ↥
Childress, Komolova, Sallee (2019): An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019 Nov;15(11):937-974. doi: 10.1080/17425255.2019.1675636. PMID: 31581854. REVIEW ↥ ↥
Heal DJ, Smith SL, Gosden J, Nutt DJ (2013): Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol. 2013 Jun;27(6):479-96. doi: 10.1177/0269881113482532. PMID: 23539642; PMCID: PMC3666194. REVIEW ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
Edel, Vollmoeller (2006): Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung bei Erwachsenen, Springer, Seite 57 ↥ ↥
Stahl (2013): Stahl’s Essential Psychopharmacology, 4. Auflage, Chapter 12: Attention deficit hyperactivity disorder and its treatment, Seite 491 ↥ ↥ ↥ ↥ ↥
Castellanos, Tannock (2002): Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes; Article in Nature reviews Neuroscience 3(8):617-28 · September 2002; DOI: 10.1038/nrn896, Seite 621 ↥
Davis, Hernandez, Stock (2020): Adolescent Polypharmacy and Serotonin Syndrome. Clin Neuropharmacol. 2020 Jan/Feb;43(1):28-30. doi: 10.1097/WNF.0000000000000375. PMID: 31934921. ↥ ↥ ↥
Prox-Vagedes, Ohlmeier in Ohlmeier, Roy (Hrsg.) (2012): ADHS bei Erwachsenen – Ein Leben in Extremen, Kapitel 5: Die Suche nach dem Rausch: Substanzabhängigkeit bei ADHS Seite 101, mwNw ↥
Kämmerer W (2024): Comparative pharmacology and abuse potential of oral dexamphetamine and lisdexamfetamine-A literature review. Hum Psychopharmacol. 2024 Jul 18:e2910. doi: 10.1002/hup.2910. PMID: 39024047. REVIEW ↥ ↥ ↥
Sharman J, Pennick M (2023): Lisdexamfetamine prodrug activation by peptidase-mediated hydrolysis in the cytosol of red blood cells. Neuropsychiatr Dis Treat. 2014 Nov 28;10:2275-80. doi: 10.2147/NDT.S70382. PMID: 25489246; PMCID: PMC4257105. ↥
https://www.adhspedia.de/wiki/Umrechnungstabelle_Medikamente ↥
Stutzman DL, Dopheide JA (2024): Practice Pearls for Stimulant Treatment of Attention-Deficit/Hyperactivity Disorder in Youth. J Pediatr Pharmacol Ther. 2024 Jun;29(3):215-231. doi: 10.5863/1551-6776-29.3.215. PMID: 38863854; PMCID: PMC11163912. ↥
Pharmazeutische Zeitung: Lisdexamfetamin|Elvanse®|71|2013 ↥ ↥
Childress, Komolova, Sallee (2019): An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019 Nov;15(11):937-974. doi: 10.1080/17425255.2019.1675636. PMID: 31581854. ↥ ↥ ↥ ↥
Heal DJ, Gosden J, Smith SL (2024): Stimulant prodrugs: A pharmacological and clinical assessment of their role in treating ADHD and binge-eating disorder. Adv Pharmacol. 2024;99:251-286. doi: 10.1016/bs.apha.2023.10.002. PMID: 38467483. REVIEW ↥
Medikamio.com: Elvanse Abruf 09.07.2023 ↥
Patel P, Marwaha R, Molla M (2025): Dextroamphetamine-Amphetamine. 2025 Apr 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 29939585. ↥ ↥ ↥ ↥
Krishnan SM, Stark JG (2008): Multiple daily-dose pharmacokinetics of lisdexamfetamine dimesylate in healthy adult volunteers. Curr Med Res Opin. 2008 Jan;24(1):33-40. doi: 10.1185/030079908x242737. PMID: 18021493. ↥
Easton N, Steward C, Marshall F, Fone K, Marsden C (2007): Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology. 2007 Feb;52(2):405-14. doi: 10.1016/j.neuropharm.2006.07.035. PMID: 17020775. ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
Huberman (2023): Adderall, Stimulants & Modafinil for ADHD: Short- & Long-Term Effects | Huberman Lab Podcast, english ↥
Mohammadi M, Akhondzadeh S (2011): Advances and considerations in attention-deficit/hyperactivity disorder pharmacotherapy. Acta Med Iran. 2011;49(8):487-98. PMID: 22009816. REVIEW ↥
Buoli, Serati, Cahn (2016): Alternative pharmacological strategies for adult ADHD treatment: a systematic review. Expert Rev Neurother. 2016;16(2):131-44. doi: 10.1586/14737175.2016.1135735. PMID: 26693882. REVIEW ↥ ↥ ↥
Fouladvand, Hankosky, Bush, Chen, Dwoskin, Freeman, Henderson, Kantak, Talbert, Tao, Zhang (2019): Predicting substance use disorder using long-term attention deficit hyperactivity disorder medication records in Truven. Health Informatics J. 2019 May 19:1460458219844075. doi: 10.1177/1460458219844075. ↥
Heal, Cheetham, Smith (2009): The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009 Dec;57(7-8):608-18. doi: 10.1016/j.neuropharm.2009.08.020. ↥ ↥ ↥ ↥ ↥ ↥ ↥
Faraone (2018): The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018 Apr;87:255-270. doi: 10.1016/j.neubiorev.2018.02.001. PMID: 29428394. ↥ ↥ ↥
Visentin R, Dalla Man C, Kovatchev B, Cobelli C (2014): The university of Virginia/Padova type 1 diabetes simulator matches the glucose traces of a clinical trial. Diabetes Technol Ther. 2014 Jul;16(7):428-34. doi: 10.1089/dia.2013.0377. PMID: 24571584; PMCID: PMC4074748. ↥
Gutiérrez-Casares JR, Quintero J, Segú-Vergés C, Rodríguez Monterde P, Pozo-Rubio T, Coma M, Montoto C (2023): In silico clinical trial evaluating lisdexamfetamine’s and methylphenidate’s mechanism of action computational models in an attention-deficit/hyperactivity disorder virtual patients’ population. Front Psychiatry. 2023 Jun 2;14:939650. doi: 10.3389/fpsyt.2023.939650. PMID: 37333910; PMCID: PMC10273406. ↥
Seeman P, Madras BK (1998): Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998 Sep;3(5):386-96. doi: 10.1038/sj.mp.4000421. PMID: 9774771. REVIEW ↥ ↥ ↥ ↥ ↥ ↥
Covey DP, Juliano SA, Garris PA (2013): Amphetamine elicits opposing actions on readily releasable and reserve pools for dopamine. PLoS One. 2013 May 3;8(5):e60763. doi: 10.1371/journal.pone.0060763. PMID: 23671560; PMCID: PMC3643976. ↥ ↥ ↥
Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D (2001): Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001 Aug 15;21(16):5916-24. doi: 10.1523/JNEUROSCI.21-16-05916.2001. PMID: 11487614; PMCID: PMC6763160. ↥ ↥
Sulzer D, Rayport S (1990): Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990 Dec;5(6):797-808. doi: 10.1016/0896-6273(90)90339-h. PMID: 2268433. ↥ ↥
Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002 Sep 15;22(18):8002-9. doi: 10.1523/JNEUROSCI.22-18-08002.2002. PMID: 12223553; PMCID: PMC6758092. ↥ ↥ ↥
Frölich, Banaschewski, Spanagel, Döpfner, Lehmkuhl (2012): Die medikamentöse Behandlung der Aufmerksamkeitsdefizit-Hyperaktivitätsstörung im Kindes- und Jugendalter mit Amphetaminpräparaten; Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie (2012), 40, pp. 287-300. https://doi.org/10.1024/1422-4917/a000185 ↥ ↥ ↥
Stahl (2013): Stahl’s Essential Psychopharmacology, 4. Auflage, Chapter 12: Attention deficit hyperactivity disorder and its treatment, Seite 490 ↥ ↥ ↥ ↥ ↥
Steinhausen, Rothenberger, Döpfner (2010): Handbuch ADHS, Seite 84, 85 ↥ ↥ ↥
Hodgkins P, Shaw M, Coghill D, Hechtman L (2012): Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry. 2012 Sep;21(9):477-92. doi: 10.1007/s00787-012-0286-5. PMID: 22763750; PMCID: PMC3432777. REVIEW ↥ ↥ ↥
Castellanos, Tannock (2002): Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes; Article in Nature reviews Neuroscience 3(8):617-28 · September 2002; DOI: 10.1038/nrn896, Seite 621, mwNw ↥ ↥
Khoshbouei H, Sen N, Guptaroy B, Johnson L’, Lund D, Gnegy ME, Galli A, Javitch JA (2004): N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004 Mar;2(3):E78. doi: 10.1371/journal.pbio.0020078. PMID: 15024426; PMCID: PMC368172. ↥ ↥ ↥
Daniels GM, Amara SG (1999):. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999 Dec 10;274(50):35794-801. doi: 10.1074/jbc.274.50.35794. PMID: 10585462. ↥
Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U (2003): N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem. 2003 Feb 14;278(7):4990-5000. doi: 10.1074/jbc.M205058200. PMID: 12464618. ↥
Giambalvo CT (2003): Differential effects of amphetamine transport vs. dopamine reverse transport on particulate PKC activity in striatal synaptoneurosomes. Synapse. 2003 Aug;49(2):125-33. doi: 10.1002/syn.10223. PMID: 12740868. ↥
Foster JD, Pananusorn B, Vaughan RA (2002): Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002 Jul 12;277(28):25178-86. doi: 10.1074/jbc.M200294200. PMID: 11994276. ↥
Mulvihill (2019): Presynaptic regulation of dopamine release: Role of the DAT and VMAT2 transporters. Neurochem Int. 2019 Jan;122:94-105. doi: 10.1016/j.neuint.2018.11.004. PMID: 30465801. ↥
Robertson SD, Matthies HJ, Galli A (2009): A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009 Apr;39(2):73-80. doi: 10.1007/s12035-009-8053-4. PMID: 19199083; PMCID: PMC2729543. REVIEW ↥
Edinoff, Akuly, Wagner, Boudreaux, Kaplan, Yusuf, Neuchat, Cornett, Boyer, Kaye, Kaye (2021): Viloxazine in the Treatment of Attention Deficit Hyperactivity Disorder. Front Psychiatry. 2021 Dec 17;12:789982. doi: 10.3389/fpsyt.2021.789982. PMID: 34975586; PMCID: PMC8718796., REVIEW ↥ ↥
Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF (2013): Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013 Jan 9;33(2):452-63. doi: 10.1523/JNEUROSCI.2136-12.2013. PMID: 23303926; PMCID: PMC3711765. ↥
Mergy MA, Gowrishankar R, Davis GL, Jessen TN, Wright J, Stanwood GD, Hahn MK, Blakely RD (2014): Genetic targeting of the amphetamine and methylphenidate-sensitive dopamine transporter: on the path to an animal model of attention-deficit hyperactivity disorder. Neurochem Int. 2014 Jul;73:56-70. doi: 10.1016/j.neuint.2013.11.009. PMID: 24332984; PMCID: PMC4177817. ↥
Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A (2000): Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6850-5. doi: 10.1073/pnas.110035297. PMID: 10823899; PMCID: PMC18764. ↥
Mulvihill (2019): Presynaptic regulation of dopamine release: Role of the DAT and VMAT2 transporters. Neurochem Int. 2019 Jan;122:94-105. doi: 10.1016/j.neuint.2018.11.004. PMID: 30465801. REVIEW ↥
Quansah, Ruiz-Rodado, Grootveld, Zetterström (2018): Methylphenidate alters monoaminergic and metabolic pathways in the cerebellum of adolescent rats. Eur Neuropsychopharmacol. 2018 Feb 22. pii: S0924-977X(18)30043-9. doi: 10.1016/j.euroneuro.2018.02.002. ↥
Quansah, Zetterström (2019): Chronic methylphenidate preferentially alters catecholamine protein targets in the parietal cortex and ventral striatum. Neurochem Int. 2019 Jan 17;124:193-199. doi: 10.1016/j.neuint.2019.01.016. ↥
Felmer AC, Janson MT, Summers KE, Wallace LJ (2019):. Extracellular dopamine kinetic parameters consistent with amphetamine effects. Synapse. 2019 Dec;73(12):e22129. doi: 10.1002/syn.22129. PMID: 31449701. ↥
Jones SR, Gainetdinov RR, Wightman RM, Caron MG (1998): Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998 Mar 15;18(6):1979-86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. PMID: 9482784; PMCID: PMC6792915. ↥
Müller, Candrian, Kropotov (2011), ADHS – Neurodiagnostik in der Praxis, Springer, Seiten 21, 86 ↥ ↥
Ruskin DN, Bergstrom DA, Shenker A, Freeman LE, Baek D, Walters JR (2001): Drugs used in the treatment of attention-deficit/hyperactivity disorder affect postsynaptic firing rate and oscillation without preferential dopamine autoreceptor action. Biol Psychiatry. 2001 Feb 15;49(4):340-50. doi: 10.1016/s0006-3223(00)00987-2. PMID: 11239905. ↥ ↥
Seeman P, Madras BK (1998): Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998 Sep;3(5):386-96. doi: 10.1038/sj.mp.4000421. PMID: 9774771. REVIEW ↥ ↥ ↥ ↥
Janenaite, Vengeliene, Bespalov, Behl (2017): Potential role of tyrosine hydroxylase in the loss of psychostimulant effect of amphetamine under conditions of impaired dopamine transporter activity. Behav Brain Res. 2017 Sep 15;334:105-108. doi: 10.1016/j.bbr.2017.07.028. PMID: 28750831. ↥
Rebec GV, Zimmerman KS (1980): Opposite effects of D-amphetamine on spontaneous neuronal activity in the neostriatum and nucleus accumbens. Brain Res. 1980 Nov 17;201(2):485-91. doi: 10.1016/0006-8993(80)91058-6. PMID: 7191347. ↥ ↥ ↥ ↥
Hansen EL, McKenzie GM (1979): Dexamphetamine increases striatal neuronal firing in freely moving rats. Neuropharmacology. 1979 Jun;18(6):547-52. doi: 10.1016/0028-3908(79)90099-6. PMID: 573376. ↥
Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS (2000): Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000 May 1;20(9):3504-11. doi: 10.1523/JNEUROSCI.20-09-03504.2000. PMID: 10777813; PMCID: PMC6773133. ↥ ↥
Newcorn JH, Ivanov I, Krone B, Li X, Duhoux S, White S, Schulz KP, Bédard AV, Pedraza J, Adler L, Blair RJ (2023): Neurobiological basis of reinforcement-based decision making in adults with ADHD treated with lisdexamfetamine dimesylate: Preliminary findings and implications for mechanisms influencing clinical improvement. J Psychiatr Res. 2023 Dec 3;170:19-26. doi: 10.1016/j.jpsychires.2023.11.037. PMID: 38101205. ↥
Schiffer WK, Volkow ND, Fowler JS, Alexoff DL, Logan J, Dewey SL (2006): Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse. 2006 Mar 15;59(4):243-51. doi: 10.1002/syn.20235. PMID: 16385551. ↥ ↥
Marinelli M, McCutcheon JE (2014): Heterogeneity of dopamine neuron activity across traits and states. Neuroscience. 2014 Dec 12;282:176-97. doi: 10.1016/j.neuroscience.2014.07.034. PMID: 25084048; PMCID: PMC4312268. REVIEW ↥
Paladini CA, Fiorillo CD, Morikawa H, Williams JT (2001): Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001 Mar;4(3):275-81. doi: 10.1038/85124. PMID: 11224544. ↥
Howlett DR, Nahorski SR (1979): Acute and chronic amphetamine treatments modulate striatal dopamine receptor binding sites. Brain Res. 1979 Jan 26;161(1):173-8. doi: 10.1016/0006-8993(79)90206-3. PMID: 758968. ↥ ↥
Mang (2018): 05. Monoamine 2: Amphetamin, Ritalin (ADHS), Cocain, Tricyclika, Videovorlesung. ca. bei Minute 14:50. ↥ ↥
Kuczenski R, Segal DS (1997): Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997 May;68(5):2032-7. doi: 10.1046/j.1471-4159.1997.68052032.x. Erratum in: J Neurochem 1997 Sep;69(3):1332. PMID: 9109529. ↥ ↥
Lahey (Hrsg.) (2012): Advances in Clinical Child Psychology, Band 9, S. 187, mit Verweis auf [Brown, Ebert, Hunt, Rapoport (1981): Urinary 3-methoxy-4-hydroxyphenylglycol and homovanillic acid response to d-amphetamine in hyperactive children; Biological Psychiatry, Vol 16(8), Aug 1981, 779-787 ↥
Shekim, Dekirmenjian, Chapel, (1979): Urinary MHPG excretion in minimal brain dysfunction and its modification by d-amphetamine; The American Journal of Psychiatry, Vol 136(5), May 1979, 667-671. http://dx.doi.org/10.1176/ajp.136.5.667 ↥
Shen, Wang (1984): Urinary 3-methoxy-4-hydroxyphenylglycol sulfate excretion in seventy-three schoolchildren with minimal brain dysfunction syndrome.Biological Psychiatry [1984, 19(6):861-870]; PMID:6743722 ↥
Lahey (Hrsg.) (2012): Advances in Clinical Child Psychology, Band 9, S. 187, mit Verweis u.a. auf Shekim, Dekirmenjian, Chapel, (1979): Urinary MHPG excretion in minimal brain dysfunction and its modification by d-amphetamine; The American Journal of Psychiatry, Vol 136(5), May 1979, 667-671. http://dx.doi.org/10.1176/ajp.136.5.667 ↥
Paladini CA, Williams JT (2004): Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004 May 12;24(19):4568-75. doi: 10.1523/JNEUROSCI.5735-03.2004. PMID: 15140928; PMCID: PMC6729397. ↥
Shi WX, Zhang XY, Pun CL, Bunney BS (2007): Clozapine blocks D-amphetamine-induced excitation of dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2007 Sep;32(9):1922-8. doi: 10.1038/sj.npp.1301334. PMID: 17299514. ↥
Shen, Shi (2020): Amphetamine promotes cortical Up state: Role of adrenergic receptors. Addict Biol. 2020 Jan 31;e12879. doi: 10.1111/adb.12879. PMID: 32003119. ↥
Shen, Shi (2021): Amphetamine Promotes Cortical Up State in Part Via Dopamine Receptors. Front Pharmacol. 2021 Aug 19;12:728729. doi: 10.3389/fphar.2021.728729. PMID: 34489713; PMCID: PMC8417369. ↥ ↥
Mang (2018): 05. Monoamine 2: Amphetamin, Ritalin (ADHS), Cocain, Tricyclika, Videovorlesung. ca. bei Minute 14:30. ↥ ↥ ↥
Heal DJ, Smith SL, Kulkarni RS, Rowley HL (2008): New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacol Biochem Behav. 2008 Aug;90(2):184-97. doi: 10.1016/j.pbb.2008.03.016. PMID: 18456311. ↥ ↥
Castellanos FX, Acosta MT (2011): Hacia un entendimiento de los mecanismos moleculares de los tratamientos farmacologicos del trastorno por deficit de atencion/hiperactividad [Towards an understanding of the molecular mechanisms underlying the pharmacological treatments of attention deficit hyperactivity disorder]. Rev Neurol. 2011 Mar 1;52 Suppl 1:S155-60. Spanish. PMID: 21365598. REVIEW ↥
Heinz (2000, 2013): Das dopaminerge Verstärkungssystem – Funktion, Interaktion mit anderen Neurotransmittersystemen und psychopathologische Korrelate, Seite 61 ↥
Callaway, Johnson, Gold, Nichols, Geyer (1991): Amphetamine derivatives induce locomotor hyperactivity by acting as indirect serotonin agonists; Psychopharmacology (Berl). 1991;104(3):293-301 ↥ ↥
Tsuchida, Kubo, Shintani, Abe, Köves, Uetsuki, Kuroda, Hashimoto, Baba (2009): Inhibitory effects of osemozotan, a serotonin 1A-receptor agonist, on methamphetamine-induced c-Fos expression in prefrontal cortical neurons. Biol Pharm Bull. 2009 Apr;32(4):728-31. doi: 10.1248/bpb.32.728. PMID: 19336914. ↥
Strajhar, Vizeli, Patt, Dolder, Kratschmar, Liechti, Odermatt (2018): Effects of lisdexamfetamine on plasma steroid concentrations compared with d-amphetamine in healthy subjects: A randomized, double-blind, placebo-controlled study. J Steroid Biochem Mol Biol. 2019 Feb;186:212-225. doi: 10.1016/j.jsbmb.2018.10.016. PMID: 30381248. ↥ ↥ ↥
Soto, Wilcox, Zhou, Kumar, Ator, Riddle, Wong, Weed (2013): Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012 Nov;37(12):2566-79. doi: 10.1038/npp.2012.119. Erratum in: Neuropsychopharmacology. 2013 May;38(6):1141. Kumar, Anil [added]. PMID: 22805599; PMCID: PMC3473325. ↥
Mattison, Plant, Lin, Chen, Chen, Twaddle, Doerge, Slikker, Patton, Hotchkiss, Callicott, Schrader, Turner, Kesner, Vitiello, Petibone, Morris (2011): Pubertal delay in male nonhuman primates (Macaca mulatta) treated with methylphenidate. Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16301-6. doi: 10.1073/pnas.1102187108. PMID: 21930929; PMCID: PMC3182701. ↥
Tsai, Chiao, Lu, Doong, Chen, Shih, Liaw, Wang, Wang (1996): Inhibition by amphetamine of testosterone secretion through a mechanism involving an increase of cyclic AMP production in rat testes. Br J Pharmacol. 1996 Jun;118(4):984-8. doi: 10.1111/j.1476-5381.1996.tb15496.x. PMID: 8799572; PMCID: PMC1909523. ↥ ↥
Tsai, Chen, Chiao, Lu, Lin, Yeh, Lo, Kau, Wang, Wang (1997): The role of cyclic AMP production, calcium channel activation and enzyme activities in the inhibition of testosterone secretion by amphetamine. Br J Pharmacol. 1997 Nov;122(5):949-55. doi: 10.1038/sj.bjp.0701463. PMID: 9384514; PMCID: PMC1565017. ↥
Angenoorth TJF, Stankovic S, Niello M, Holy M, Brandt SD, Sitte HH, Maier J (2021): Interaction Profiles of Central Nervous System Active Drugs at Human Organic Cation Transporters 1-3 and Human Plasma Membrane Monoamine Transporter. Int J Mol Sci. 2021 Nov 30;22(23):12995. doi: 10.3390/ijms222312995. PMID: 34884800; PMCID: PMC8657792. ↥ ↥
Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S (2006): Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006 Jun;50(8):941-52. doi: 10.1016/j.neuropharm.2006.01.005. PMID: 16581093. ↥ ↥ ↥
Orrico-Sanchez A, Chausset-Boissarie L, Alves de Sousa R, Coutens B, Rezai Amin S, Vialou V, Louis F, Hessani A, Dansette PM, Zornoza T, Gruszczynski C, Giros B, Guiard BP, Acher F, Pietrancosta N, Gautron S (2020): Antidepressant efficacy of a selective organic cation transporter blocker in a mouse model of depression. Mol Psychiatry. 2020 Jun;25(6):1245-1259. doi: 10.1038/s41380-019-0548-4. PMID: 31619760. ↥
Bacq A, Balasse L, Biala G, Guiard B, Gardier AM, Schinkel A, Louis F, Vialou V, Martres MP, Chevarin C, Hamon M, Giros B, Gautron S (2012): Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry. 2012 Sep;17(9):926-39. doi: 10.1038/mp.2011.87. PMID: 21769100. ↥
Matochik, Liebenauer, King, Szymanski, Cohen, Zametkin (1994): Cerebral glucose metabolism in adults with attention defi cit hyperactivity disorder after chronic stimulant treatment. Am J Psychiatry 151: 658–664; Achtung, geringes N von 37; geringe Stichprobe mit n = 18; zitiert nach Edel, Vollmoeller (2006): Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung bei Erwachsenen, Springer, Seite 10 ↥
Stroemer, Kent, Hulsebosch (1998): Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats; Stroke. 1998 Nov;29(11):2381-93; discussion 2393-5. ↥
Rau T, Ziemniak J, Poulsen D (2016): The neuroprotective potential of low-dose methamphetamine in preclinical models of stroke and traumatic brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Jan 4;64:231-6. doi: 10.1016/j.pnpbp.2015.02.013. PMID: 25724762. REVIEW ↥
Jin C, Schachar R (2004): Methylphenidate treatment of attention-deficit/hyperactivity disorder secondary to traumatic brain injury: a critical appraisal of treatment studies. CNS Spectr. 2004 Mar;9(3):217-26. doi: 10.1017/s1092852900009019. PMID: 14999162. ↥
Takahashi, Ohmiya, Honda, Ni (2018): The KCNH3 inhibitor ASP2905 shows potential in the treatment of attention deficit/hyperactivity disorder. PLoS One. 2018 Nov 21;13(11):e0207750. doi: 10.1371/journal.pone.0207750. eCollection 2018. ↥
Hutson, Heins, Folgering (2015): Effects of lisdexamfetamine alone and in combination with s-citalopram on acetylcholine and histamine efflux in the rat pre-frontal cortex and ventral hippocampus. J Neurochem. 2015 Aug;134(4):693-703. doi: 10.1111/jnc.13157. ↥
Madras, Miller, Fischman (2005): The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005 Jun 1;57(11):1397-409. doi: 10.1016/j.biopsych.2004.10.011. PMID: 15950014. ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
Nagy, Häge, Coghill, Caballero, Adey, Anderson, Sikirica, Cardo (2015): Functional outcomes from a head-to-head, randomized, double-blind trial of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder and an inadequate response to methylphenidate.Eur Child Adolesc Psychiatry. 2016 Feb;25(2):141-9. doi: 10.1007/s00787-015-0718-0. ↥
Madhoo, Keefe, Roth, Sambunaris, Wu, Trivedi, Anderson, Lasser (2014): Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder; Neuropsychopharmacology. 2014 May;39(6):1388-98. doi: 10.1038/npp.2013.334 n = 143 Erwachsene ↥
Coghill, Banaschewski, Nagy, Otero, Soutullo, Yan, Caballero, Zuddas (2017): Long-Term Safety and Efficacy of Lisdexamfetamine Dimesylate in Children and Adolescents with ADHD: A Phase IV, 2-Year, Open-Label Study in Europe.CNS Drugs. 2017 Jun 30. doi: 10.1007/s40263-017-0443-y; n = 314 ↥ ↥
Arnold: Journal of Attention Disorders Vol. 3(4):200-211 (2000) Methylphenidate vs. amphetamine: Comparative review n = 174 ↥
Müller, Candrian, Kropotov (2011), ADHS – Neurodiagnostik in der Praxis, Seite 21 ↥
Diamond: Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention-deficit (with hyperactivity), Development and Psychopathology 17 (2005), 807–825, Seite 811 ↥
Liddle, Hollis, Batty, Groom, Totman, Liotti, Scerif, Liddle (2011): Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry. 2011 Jul;52(7):761-71. doi: 10.1111/j.1469-7610.2010.02333.x. ↥
ADHS-Chaoten;Thread: Amphetamin und Methylphenidat für Depressionen ↥
Adler, Goodman, Weisler, Hamdani, Roth (2009): Effect of lisdexamfetamine dimesylate on sleep in adults with attention-deficit/hyperactivity disorder. Behav Brain Funct. 2009 Aug 3;5:34. doi: 10.1186/1744-9081-5-34. PMID: 19650932; PMCID: PMC2732626. ↥
Giblin, Strobel (2011): Effect of lisdexamfetamine dimesylate on sleep in children with ADHD. J Atten Disord. 2011 Aug;15(6):491-8. doi: 10.1177/1087054710371195. PMID: 20574056. ↥
López FA, Leroux JR (2013): Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges. Atten Defic Hyperact Disord. 2013 Sep;5(3):249-65. doi: 10.1007/s12402-013-0106-x. PMID: 23564273; PMCID: PMC3751218. REVIEW ↥
Mattay, Goldberg, Fera, Hariri, Tessitore, Egan, Kolachana, Callicott, Weinberger (2003): Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine; doi: 10.1073/pnas.0931309100; PNAS May 13, 2003 vol. 100 no. 10 6186-6191 ↥
Childress, Newcorn, Cutler (2019): Gender Effects in the Efficacy of Racemic Amphetamine Sulfate in Children with Attention-Deficit/Hyperactivity Disorder. Adv Ther. 2019 Apr 10. doi: 10.1007/s12325-019-00942-5. ↥
Arnold (2000): L.E. Methylphenidate vs amphetamine: Comparative review, J. of Att. Disorders, 2000, Vol 3, 200ff, n = 174 ↥
Müller, Candrian, Kropotov (2011), ADHS – Neurodiagnostik in der Praxis, Springer, Seite 21 ↥
Castello et al. 1992, zitiert nach Arnold: Journal of Attention Disorders Vol. 3(4):200-211 (2000) Methylphenidate vs. amphetamine: Comparative review ↥ ↥
Brams, Weisler, Findling, Gasior, Hamdani, Ferreira-Cornwell, Squires: Maintenance of Efficacy of Lisdexamfetamine Dimesylate in Adults With Attention-Deficit/Hyperactivity Disorder: Randomized Withdrawal Design, J Clin Psychiatry 2012;73(7):977-983; 10.4088/JCP.11m07430 ↥
http://www.ads-hyperaktivitaet.de/FAQ/Infos/Medis/medis.html#1 ↥
Markowitz, Patrick (2017): The Clinical Pharmacokinetics of Amphetamines Utilized in the Treatment of Attention-Deficit/Hyperactivity Disorder. J Child Adolesc Psychopharmacol. 2017 Oct;27(8):678-689. doi: 10.1089/cap.2017.0071. PMID: 28910145. REVIEW ↥
Dolder, Strajhar, Vizeli, Hammann, Odermatt, Liechti (2017): Pharmacokinetics and Pharmacodynamics of Lisdexamfetamine Compared with D-Amphetamine in Healthy Subjects. Front Pharmacol. 2017 Sep 7;8:617. doi: 10.3389/fphar.2017.00617. PMID: 28936175; PMCID: PMC5594082. n = 24) Die Studie benennt allerdings nicht das Gewicht der Probanden, sodass keine Aussage über die Dosierung je kg Körpergewicht möglich ist. Das Gewicht ist jedoch bei der Amphetamindosierung ein relevanter Faktor.((Markowitz, Patrick (2017): The Clinical Pharmacokinetics of Amphetamines Utilized in the Treatment of Attention-Deficit/Hyperactivity Disorder. J Child Adolesc Psychopharmacol. 2017 Oct;27(8):678-689. doi: 10.1089/cap.2017.0071. PMID: 28910145. REVIEW ↥
Amphetamines. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-2016 ↥
Ito, Onodera, Yamatodani, Watanabe, Sato (1997): The effect of methamphetamine on histamine release in the rat hypothalamus. Psychiatry Clin Neurosci. 1997 Apr;51(2):79-81. doi: 10.1111/j.1440-1819.1997.tb02911.x. PMID: 9141145. ↥
Ito, Onodera, Sakurai, Sato, Watanabe (1996): The effect of methamphetamine on histamine level and histidine decarboxylase activity in the rat brain. Brain Res. 1996 Sep 23;734(1-2):98-102. PMID: 8896814. ↥
Houghton, de Vries, Loss (2019): Psychostimulants/Atomoxetine and Serious Cardiovascular Events in Children with ADHD or Autism Spectrum Disorder. CNS Drugs. 2019 Nov 25. doi: 10.1007/s40263-019-00686-4. n = 2.566.995 ↥
Forns, Dudukina, Hägg, Szentkúti, Gembert, Plana, Gilsenan, Horváth-Puhó, Ehrenstein, Reutfors, Rebordosa )2022): Risk of Major Cardiovascular and Cerebrovascular Events in Users of Lisdexamfetamine and Other Medications for Attention-Deficit/Hyperactivity Disorder in Denmark and Sweden: A Population-Based Cohort Study. Neurol Ther. 2022 Aug 26. doi: 10.1007/s40120-022-00396-y. PMID: 36028603. n = 273.000 ↥
Harris E. Long-Term ADHD Medications and Cardiovascular Disease Risk. JAMA. 2023 Dec 26;330(24):2331. doi: 10.1001/jama.2023.24173. PMID: 38055293. n = 60.000 ↥
Chan M, Chan JJ, Wright JM (2025): Effect of amphetamines on blood pressure. Cochrane Database Syst Rev. 2025 Mar 28;3(3):CD007896. doi: 10.1002/14651858.CD007896.pub4. PMID: 40152309; PMCID: PMC11951410. REVIEW ↥
Manso, Morcillo, Pereira, Maldonado (2020): Tricotilomanía de nueva aparición durante el tratamiento con fármacos estimulantes. A propósito de dos casos clínicos pediátricos [New-onset trichotillomania during treatment with stimulant drugs. About two pediatric clinical cases]. Arch Argent Pediatr. 2020 Feb;118(1):e61-e62. Spanish. doi: 10.5546/aap.2020.e61. PMID: 31984712. ↥
Abdalla TE, Kotsonis D, Best J, Ramasamy R, Wood E (2021): Stimulant-Induced Pituitary Failure and Reversible Azoospermia. Cureus. 2021 Apr 3;13(4):e14269. doi: 10.7759/cureus.14269. PMID: 33959450; PMCID: PMC8093113. ↥
Assem-Hilger, Kasper (2005): Psychopharmaka und sexuelle Dysfunktion. Journa lfür Neurologie, Neurochirurgie und Psychiatrie; 2005, 30-36 ↥
Duca Y, Aversa A, Condorelli RA, Calogero AE, La Vignera S (2019): Substance Abuse and Male Hypogonadism. J Clin Med. 2019 May 22;8(5):732. doi: 10.3390/jcm8050732. PMID: 31121993; PMCID: PMC6571549. REVIEW ↥ ↥
Strajhar P, Vizeli P, Patt M, Dolder PC, Kratschmar DV, Liechti ME, Odermatt A (2019): Effects of lisdexamfetamine on plasma steroid concentrations compared with d-amphetamine in healthy subjects: A randomized, double-blind, placebo-controlled study. J Steroid Biochem Mol Biol. 2019 Feb;186:212-225. doi: 10.1016/j.jsbmb.2018.10.016. PMID: 30381248. n = 24 ↥
Yamamoto Y, Yamamoto K, Hayase T (1999): Effect of methamphetamine on male mice fertility. J Obstet Gynaecol Res. 1999 Oct;25(5):353-8. doi: 10.1111/j.1447-0756.1999.tb01176.x. PMID: 10533332. ↥
Lin JF, Lin YH, Liao PC, Lin YC, Tsai TF, Chou KY, Chen HE, Tsai SC, Hwang TI (2014): Induction of testicular damage by daily methamphetamine administration in rats. Chin J Physiol. 2014 Feb 28;57(1):19-30. doi: 10.4077/CJP.2014.BAB155. PMID: 24621335. ↥
Kaewman P, Nudmamud-Thanoi S, Thanoi S (2018): GABAergic Alterations in the Rat Testis after Methamphetamine Exposure. Int J Med Sci. 2018 Aug 10;15(12):1349-1354. doi: 10.7150/ijms.27609. PMID: 30275762; PMCID: PMC6158670. ↥
Dickerson SM, Walker DM, Reveron ME, Duvauchelle CL, Gore AC (2008): The recreational drug ecstasy disrupts the hypothalamic-pituitary-gonadal reproductive axis in adult male rats. Neuroendocrinology. 2008;88(2):95-102. doi: 10.1159/000119691. PMID: 18309234; PMCID: PMC2753463. ↥
Barenys M, Macia N, Camps L, de Lapuente J, Gomez-Catalan J, Gonzalez-Linares J, Borras M, Rodamilans M, Llobet JM (2009): Chronic exposure to MDMA (ecstasy) increases DNA damage in sperm and alters testes histopathology in male rats. Toxicol Lett. 2009 Dec 1;191(1):40-6. doi: 10.1016/j.toxlet.2009.08.002. PMID: 19683041. ↥ ↥
Yamamoto Y, Yamamoto K, Hayase T, Abiru H, Shiota K, Mori C (2002): Methamphetamine induces apoptosis in seminiferous tubules in male mice testis. Toxicol Appl Pharmacol. 2002 Feb 1;178(3):155-60. doi: 10.1006/taap.2001.9330. PMID: 11858731. ↥
Alavi SH, Taghavi MM, Moallem SA (2008): Evaluation of effects of methamphetamine repeated dosing on proliferation and apoptosis of rat germ cells. Syst Biol Reprod Med. 2008 Mar-Apr;54(2):85-91. doi: 10.1080/19396360801952078. PMID: 18446649. ↥
Nudmamud-Thanoi S, Thanoi S (2011): Methamphetamine induces abnormal sperm morphology, low sperm concentration and apoptosis in the testis of male rats. Andrologia. 2011 Aug;43(4):278-82. doi: 10.1111/j.1439-0272.2010.01071.x. PMID: 21486410. ↥
Saberi A, Sepehri G, Safi Z, Razavi B, Jahandari F, Divsalar K, Salarkia E (2017): Effects of Methamphetamine on Testes Histopathology and Spermatogenesis Indices of Adult Male Rats. Addict Health. 2017 Fall;9(4):199-205. PMID: 30574282; PMCID: PMC6294480. ↥
Mobaraki F, Seghatoleslam M, Fazel A, Ebrahimzadeh-Bideskan A (2018): Effects of MDMA (ecstasy) on apoptosis and heat shock protein (HSP70) expression in adult rat testis. Toxicol Mech Methods. 2018 Mar;28(3):219-229. doi: 10.1080/15376516.2017.1388461. PMID: 29105552. ↥
Levine LA, Betcher HK, Ziegelmann MJ, Bajic P (2020): Amphetamine/Dextroamphetamine Salts for Delayed Orgasm and Anorgasmia in Men: A Pilot Study. Urology. 2020 Aug;142:141-145. doi: 10.1016/j.urology.2020.04.081. PMID: 32360625. n = 17 ↥
Bartlik BD, Kaplan P, Kaplan HS (1995): Psychostimulants apparently reverse sexual dysfunction secondary to selective serotonin re-uptake inhibitors. J Sex Marital Ther. 1995 Winter;21(4):264-71. doi: 10.1080/00926239508414646. PMID: 8789508. ↥
Coskun M, Zoroglu S (2009): A report of two cases of sexual side effects with OROS methylphenidate. J Child Adolesc Psychopharmacol. 2009 Aug;19(4):477-9. doi: 10.1089/cap.2008.0161. PMID: 19702503. ↥
Cakin-Memik N, Yildiz O, Sişmanlar SG, Karakaya I, Ağaoğlu B (2010): Priapism associated with methylphenidate: a case report. Turk J Pediatr. 2010 Jul-Aug;52(4):430-4. PMID: 21043394. ↥
Ostdiek-Wille GP, Bavitz KC, Kohn TP, Deibert CM (2023): Attention-deficit hyperactivity disorder medication use is associated with testosterone hypofunction-results from a national claims database analysis. Int J Impot Res. 2023 Dec 21. doi: 10.1038/s41443-023-00805-2. PMID: 38129694. n = 34.448 ↥
Ermer J, Haffey MB, Richards C, Lasseter K, Adeyi B, Corcoran M, Stanton B, Martin P (2013): Double-blind, placebo-controlled, two-period, crossover trial to examine the pharmacokinetics of lisdexamfetamine dimesylate in healthy older adults. Neuropsychiatr Dis Treat. 2013;9:219-29. doi: 10.2147/NDT.S38377. PMID: 23431065; PMCID: PMC3575217. ↥
Mang (2018): 05. Monoamine 2: Amphetamin, Ritalin (ADHS), Cocain, Tricyclika, Videovorlesung. ca. bei Minute 38. ↥
Spiller HA, Hays HL, Aleguas A Jr (2013): Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs. 2013 Jul;27(7):531-43. doi: 10.1007/s40263-013-0084-8. PMID: 23757186. REVIEW ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥ ↥
Fitzgerald KT, Bronstein AC (2013): Adderall® (amphetamine-dextroamphetamine) toxicity. Top Companion Anim Med. 2013 Feb;28(1):2-7. doi: 10.1053/j.tcam.2013.03.002. PMID: 23796480. ↥ ↥ ↥ ↥ ↥ ↥ ↥
Honório da Silva JV, Erthal RP, Vercellone IC, Santos DPD, Ferraz CR, de Matos RLN, Gonçalves LED, Bracarense APFRL, Verri WA Jr, Câmara NOS, de Andrade FG, Fernandes GSA (2024): Lisdexamfetamine dimesylate-exposition in male rats during the peripubertal period impairs inflammatory mechanisms, antioxidant activity, and apoptosis process in kidneys of male pubertal rats. J Biochem Mol Toxicol. 2024 Aug;38(8):e23781. doi: 10.1002/jbt.23781. PMID: 39051179. ↥
Sharman J, Pennick M (2014): Lisdexamfetamine prodrug activation by peptidase-mediated hydrolysis in the cytosol of red blood cells. Neuropsychiatr Dis Treat. 2014 Nov 28;10:2275-80. doi: 10.2147/NDT.S70382. PMID: 25489246; PMCID: PMC4257105. ↥
Ward K, Citrome L (2018): Lisdexamfetamine: chemistry, pharmacodynamics, pharmacokinetics, and clinical efficacy, safety, and tolerability in the treatment of binge eating disorder. Expert Opin Drug Metab Toxicol. 2018 Feb;14(2):229-238. doi: 10.1080/17425255.2018.1420163. PMID: 29258368. REVIEW ↥ ↥
Gerlach S, Maruf AA, Shaheen SM, McCloud R, Heintz M, McAusland L, Arnold PD, Bousman CA (2024): Effect of CYP2D6 genetic variation on patient-reported symptom improvement and side effects among children and adolescents treated with amphetamines. Pharmacogenet Genomics. 2024 Jul 1;34(5):149-153. doi: 10.1097/FPC.0000000000000529. PMID: 38517706. n = 214 ↥
Law R, Lewis D, Hain D, Daut R, DelBello MP, Frazier JA, Newcorn JH, Nurmi E, Cogan ES, Wagner S, Johnson H, Lanchbury J (2022): Characterisation of seven medications approved for attention-deficit/hyperactivity disorder using in vitro models of hepatic metabolism. Xenobiotica. 2022 Nov 1:1-32. doi: 10.1080/00498254.2022.2141151. PMID: 36317558. ↥
Lin LY, Di Stefano EW, Schmitz DA, Hsu L, Ellis SW, Lennard MS, Tucker GT, Cho AK (1997): Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos. 1997 Sep;25(9):1059-64. PMID: 9311621. ↥
Bach MV, Coutts RT, Baker GB (1999): Involvement of CYP2D6 in the in vitro metabolism of amphetamine, two N-alkylamphetamines and their 4-methoxylated derivatives. Xenobiotica. 1999 Jul;29(7):719-32. doi: 10.1080/004982599238344. PMID: 10456690. ↥
Wu D, Otton SV, Inaba T, Kalow W, Sellers EM (1997): Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997 Jun 1;53(11):1605-12. doi: 10.1016/s0006-2952(97)00014-2. PMID: 9264312. ↥ ↥
de la Torre R, Yubero-Lahoz S, Pardo-Lozano R, Farré M (2012): MDMA, methamphetamine, and CYP2D6 pharmacogenetics: what is clinically relevant? Front Genet. 2012 Nov 12;3:235. doi: 10.3389/fgene.2012.00235. PMID: 23162568; PMCID: PMC3495276. ↥
Phamarkogenetik.de: Cytochrom P450 2D6 (CYP2D6) [T88.7] Abruf 25.03.2022 ↥ ↥
Russell DJ, Wyrwoll CS, Preen DB, Kelty E (2024): Investigating maternal and neonatal health outcomes associated with continuing or ceasing dexamphetamine treatment for women with attention-deficit hyperactivity disorder during pregnancy: a retrospective cohort study. Arch Womens Ment Health. 2024 Mar 1. doi: 10.1007/s00737-024-01450-4. PMID: 38424254. ↥
Suarez EA, Bateman BT, Hernandez-Diaz S, Straub L, McDougle CJ, Wisner KL, Gray KJ, Pennell PB, Lester B, Zhu Y, Mogun H, Huybrechts KF (2024): Prescription Stimulant Use During Pregnancy and Risk of Neurodevelopmental Disorders in Children. JAMA Psychiatry. 2024 Jan 24:e235073. doi: 10.1001/jamapsychiatry.2023.5073. PMID: 38265792; PMCID: PMC10809143. n = 4,3 Mio ↥
Bang Madsen K, Larsson H, Skoglund C, Liu X, Munk-Olsen T, Bergink V, Newcorn JH, Cortese S, Lichtenstein P, Kuja-Halkola R, Chang Z, D’Onofrio B, Hove Thomsen P, Klungsøyr K, Brikell I, Garcia-Argibay M (2025): In utero exposure to methylphenidate, amphetamines and atomoxetine and offspring neurodevelopmental disorders - a population-based cohort study and meta-analysis. Mol Psychiatry. 2025 Mar 27. doi: 10.1038/s41380-025-02968-4. PMID: 40148550. ↥
Rose, Hathcock, White, Borowski, Rivera-Chiauzzi (2020): Amphetamine-Dextroamphetamine and Pregnancy: Neonatal Outcomes After Prenatal Prescription Mixed Amphetamine Exposure. J Atten Disord. 2020 Jan 13:1087054719896857. doi: 10.1177/1087054719896857. ↥
Bro, Kjaersgaard, Parner, Sørensen, Olsen, Bech, Pedersen, Christensen, Vestergaard (2015): Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy. Clin Epidemiol. 2015 Jan 29;7:139-47. doi: 10.2147/CLEP.S72906. eCollection 2015. ↥
Cohen, Hernández-Díaz, Bateman, Park, Desai, Gray, Patorno, Mogun, Huybrechts (2017): Placental Complications Associated With Psychostimulant Use in Pregnancy. Obstet Gynecol. 2017 Dec;130(6):1192-1201. doi: 10.1097/AOG.0000000000002362. ↥
Haervig, Mortensen, Hansen, Strandberg-Larsen (2014): Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemiol Drug Saf. 2014 May;23(5):526-33. doi: 10.1002/pds.3600. n = 480 unter 1.054.494 Geburten ↥
Pottegård, Hallas, Andersen, Løkkegaard, Dideriksen, Aagaard, Damkier (2014): First-trimester exposure to methylphenidate: a population-based cohort study. J Clin Psychiatry. 2014 Jan;75(1):e88-93. doi: 10.4088/JCP.13m08708. ↥
Szpunar MJ, Freeman MP, Kobylski LA, Rossa ET, Gaccione P, Chitayat D, Viguera AC, Cohen LS (2023): Risk of Major Malformations in Infants After First-Trimester Exposure to Stimulants: Results From the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications. J Clin Psychopharmacol. 2023 Jul-Aug 01;43(4):326-332. doi: 10.1097/JCP.0000000000001702. PMID: 37235505. ↥
Moran, Ongur, Hsu, Castro, Perlis, Schneeweiss (2019): Psychosis with Methylphenidate or Amphetamine in Patients with ADHD. N Engl J Med. 2019 Mar 21;380(12):1128-1138. doi: 10.1056/NEJMoa1813751. n = 221.846 ↥
Jongsma, Gayer-Anderson, Lasalvia, Quattrone, Mulè, Szöke, Selten, Turner, Arango, Tarricone, Berardi, Tortelli, Llorca, de Haan, Bobes, Bernardo, Sanjuán, Santos, Arrojo, Del-Ben, Menezes, Velthorst, Murray, Rutten, Jones, van Os, Morgan, Kirkbride; for the European Network of National Schizophrenia Networks Studying Gene-Environment Interactions Work Package 2 (EU-GEI WP2) Group(2018): Treated Incidence of Psychotic Disorders in the Multinational EU-GEI Study. JAMA Psychiatry. 2018;75(1):36–46. doi:10.1001/jamapsychiatry.2017.3554; n = 12,9 Millionen Personenjahre ↥
Gudbrandsdottir RK, Sigurdsson E, Albertsson ÞI, Jonsdottir H, Ingimarsson O (2025): Risk of hospitalisation for first-onset psychosis or mania within a year of ADHD medication initiation in adults with ADHD. BMJ Ment Health. 2025 Apr 12;28(1):e301521. doi: 10.1136/bmjment-2024-301521. PMID: 40221142; PMCID: PMC11997813. ↥
Gelbe Liste: Dexamfetamin. Abgerufen 12.02.23 ↥ ↥ ↥
Cosgrove K, Johnston CS (2017): Examining the Impact of Adherence to a Vegan Diet on Acid-Base Balance in Healthy Adults. Plant Foods Hum Nutr. 2017 Sep;72(3):308-313. doi: 10.1007/s11130-017-0620-7. PMID: 28677099. ↥
Osuna-Padilla IA, Leal-Escobar G, Garza-García CA, Rodríguez-Castellanos FE (2019): Dietary Acid Load: mechanisms and evidence of its health repercussions. Nefrologia (Engl Ed). 2019 Jul-Aug;39(4):343-354. English, Spanish. doi: 10.1016/j.nefro.2018.10.005. PMID: 30737117. REVIEW ↥
Passey C (2017): Reducing the Dietary Acid Load: How a More Alkaline Diet Benefits Patients With Chronic Kidney Disease. J Ren Nutr. 2017 May;27(3):151-160. doi: 10.1053/j.jrn.2016.11.006. PMID: 28117137 REVIEW ↥
Terrillion CE, Dao DT, Cachope R, Lobo MK, Puche AC, Cheer JF, Gould TD (2017): Reduced levels of Cacna1c attenuate mesolimbic dopamine system function. Genes Brain Behav. 2017 Jun;16(5):495-505. doi: 10.1111/gbb.12371. PMID: 28186690; PMCID: PMC5457318. ↥
Schoretsanitis, de Leon, Eap, Kane, Paulzen (2019): Clinically Significant Drug-Drug Interactions with Agents for Attention-Deficit/Hyperactivity Disorder. CNS Drugs. 2019 Dec;33(12):1201-1222. doi: 10.1007/s40263-019-00683-7. ↥ ↥ ↥
Pharmazeutische Zeitung: Lisdexamfetamin; abgerufen 10.02.21 ↥ ↥ ↥ ↥
Benassayag Kaduri N, Hazan A, De-Haan T, Kohn E, Berkovitch M, Berlin M (2024): Amphetamine use for attention deficit hyperactive disorder during breastfeeding and children’s neurodevelopmental outcomes: A pilot study. Psychiatry Res. 2024 Jun 17;339:116047. doi: 10.1016/j.psychres.2024.116047. PMID: 38908263. ↥
Castells, Ramon, Cunill, Olivé, Serrano (2020): Relationship Between Treatment Duration and Efficacy of Pharmacological Treatment for ADHD: A Meta-Analysis and Meta-Regression of 87 Randomized Controlled Clinical Trials. J Atten Disord. 2020 Feb 20:1087054720903372. doi: 10.1177/1087054720903372. PMID: 32075485. ↥
Schoeman R (2024): MEDICAL MANAGEMENT OF ADHD: THE LATEST TREATMENT AND MEDICATION OPTIONS FOR PATIENTS; MHM 2024, Volume 11, Issue 1 ↥
