The autonomic nervous system: sympathetic / parasympathetic nervous system
The autonomic nervous system (ANS) consists of two parts, the (more activating) sympathetic nervous system and the (more inhibiting) parasympathetic nervous system. These two systems form a dynamic balance.
The sympathetic and parasympathetic nervous systems are controlled by different regions of the brain. The sympathetic nervous system is activated by the paraventricular nucleus, the locus coeruleus and the ventrolateral medulla. The parasympathetic nervous system is controlled by the nucleus tractus solitarius, the dorsal motor nucleus of the vagus nerve and the nucleus ambiguus.
The autonomic nervous system is semi-autonomous, i.e. many reactions are controlled directly in the spinal cord without the involvement of the brain, while others are regulated by higher-level instances (hypothalamus, brain stem, limbic system).
The sympathetic and parasympathetic nervous systems are not rigidly connected like a seesaw, but can be active or passive independently of each other.
Most of the organs of fulfillment are connected to the sympathetic and parasympathetic nervous system via direct nerves. They are controlled preganglionically via acetylcholine and postganglionically via noradrenaline. Depending on the organ, both neurotransmitters have an inhibitory or stimulating effect.
In stressful situations, the sympathetic nervous system reacts by activating organs and increasing the heart rate, breathing and blood pressure. Consequences include increased alertness and increased flight behavior. The alpha-amylase level in saliva is considered a biomarker of the sympathetic nervous system.
The activity of the autonomic nervous system can be determined by measuring heart rate variability.
A meta-analysis of 55 studies on the VNS in ADHD found no influence of the VNS on ADHD in almost half of the studies. Nevertheless, stimulants and rewards do influence the VNS.1
In ADHD, adrenaline levels are reduced and the parasympathetic nervous system is excessive and inflexible. The findings on the sympathetic nervous system in ADHD are inconsistent. ADHD medication can cause changes in the autonomic nervous system and almost balance the activity of the sympathetic and parasympathetic nervous systems.
- 1. Sympathetic nervous system (activating)
- 2. Parasympathetic nervous system, vagus (inhibitory)
- 3. Control of the sympathetic, parasympathetic and HPA axis
- 4. Stress reaction of the autonomic nervous system
- 5. Alpha-amylase as a biomarker of the autonomic nervous system
- 6. Measurement of the autonomic nervous system using heart rate variability (HRV)
- 7. Vegetative nervous system and ADHD
- 8. Changes in the autonomic nervous system due to ADHD medication
- 9. Parasympathetic / sympathetic nervous system for other disorders
1. Sympathetic nervous system (activating)
The sympathetic nervous system promotes motivation, activates and alerts.
Its nerves lead from the brain stem to the thoracic and lumbar parts of the spinal cord.
The sympathetic nervous system is a network of the brain regions2
- PVN, paraventricular nucleus / nucleus paraventricularis
- A nucleus of the hypothalamus
- Produced
- Oxytocin
- Antidiuretic hormone (low)
- CRH
- Locus coeruleus
- Produces noradrenaline
- Ventrolateral medulla
- Produces noradrenaline
- Regulated
- Arterial blood pressure
- Breathing
The transmitter control of the sympathetic nervous system takes place preganglionically (up to the ganglion) via acetylcholine.
Binding to cholinoceptors:
- N-receptors (nicotinergic)
Transmitter control postganglionically (from the ganglion) is via noradrenaline.
Binding to adrenoreceptors:
- Alpha receptors
- Beta receptors
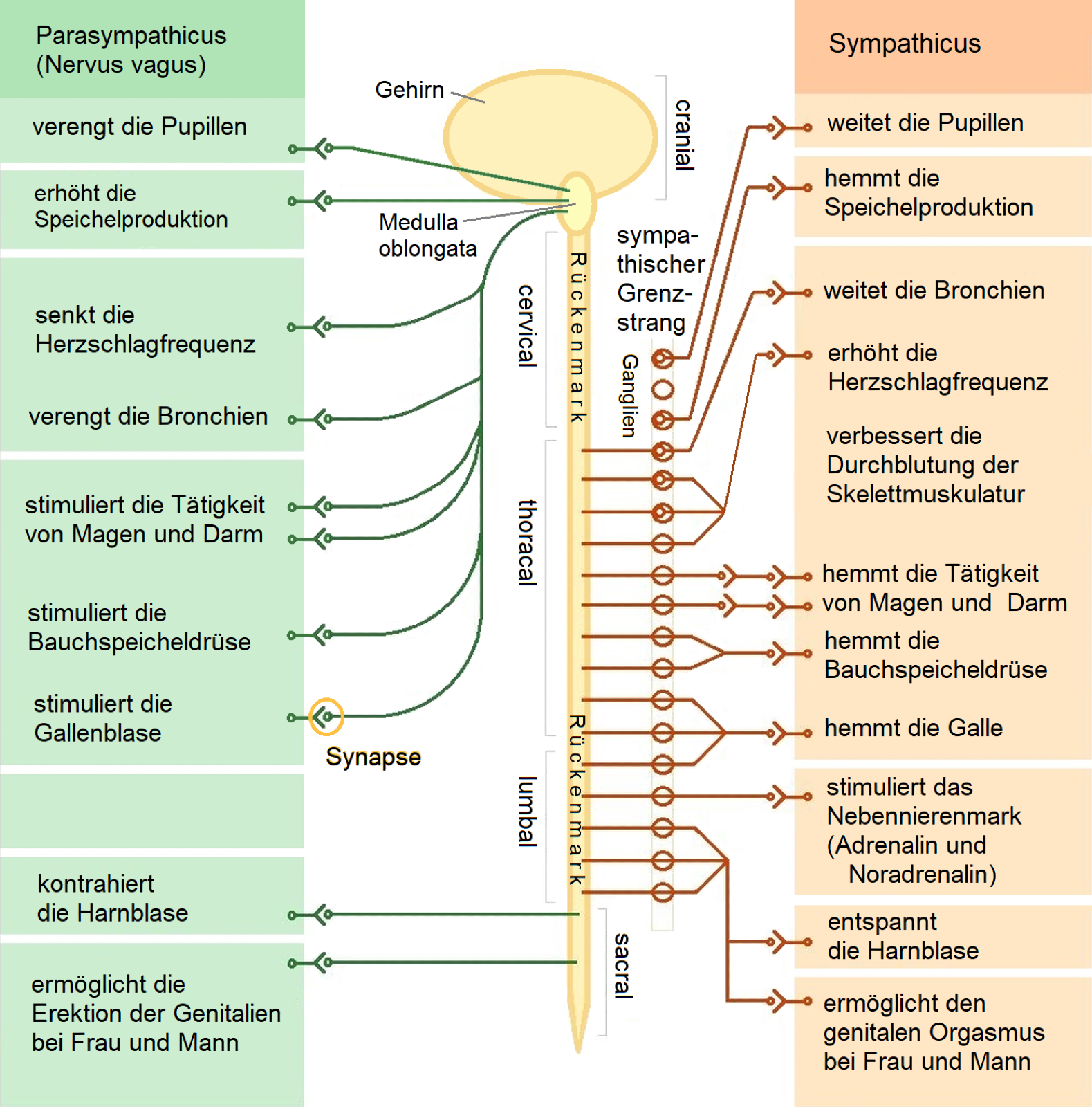
Graphic by : Sciencia58, CC BY-SA 3.0, File: The autonomic nervous system.png - Wikimedia Commons
2. Parasympathetic nervous system, vagus (inhibitory)
The parasympathetic nervous system inhibits motivation, calms and aids digestion.
Its nerves lead from the brain stem through the cranial nerves and the sacral area of the spinal cord through the spinal cord nerves.
The parasympathetic nervous system is a network of the brain regions2
- NTS, nucleus tractus solitarius
- Controls
- Taste perception (“taste nucleus”)
- Respiratory reflex
- Gag reflex
- Emetic reflex
- Controls
- DMX, nucleus dorsalis nervi vagi, dorsal motor nucleus of the vagus nerve
- Part of the medulla oblongata
- NA, Nucleus ambiguus
- Part of the medulla oblongata
Transmitter control of the parasympathetic nervous system is carried out preganglionically (up to the ganglion) and postganglionically (from the ganglion) by acetylcholine.
Binding to cholinoceptors
- N-receptors (nicotinergic)
- M receptors (muscarinergic)
3. Control of the sympathetic, parasympathetic and HPA axis
The hypothalamus and brain stem moderate the actions of the sympathetic and parasympathetic nervous system in order to maintain the body’s constantly changing conditions in what is known as homeostatic equilibrium.
While the HPA axis is controlled by neurotransmitters and hormones (endocrine), the autonomic nervous system is controlled neuronally (electrically). This is why the response of the autonomic nervous system is much faster.
3.1. Activation of the sympathetic nervous system
- Amygdala →
and - Intralimbic cortex →
- → Nucleus of the solitary tract →
- → Locus coeruleus →
- → Sympathetic nervous system
- → ventrolateral medulla →
- → Sympathetic nervous system
- → Hypothalamus (there: paraventricular nucleus) →
- → Sympathetic nervous system
- → Locus coeruleus →
- → Nucleus of the solitary tract →
- Dorsomedial hypothalamus →
- → Hypothalamus (there: paraventricular nucleus) →
- → Sympathetic nervous system
- → Hypothalamus (there: paraventricular nucleus) →
Source3
3.2. Activation of the parasympathetic nervous system
- Stria terminalis (there: anterior bed nucleus) →
- → Hypothalamus (there: paraventricular nucleus) →
- → dorsal motor nucleus of the vagus nerve →
- → Parasympathetic nervous system
- → dorsal motor nucleus of the vagus nerve →
- → Nucleus of the solitary tract →
- → dorsal motor nucleus of the vagus nerve →
- → Parasympathetic nervous system
- → Nucleus ambiguus →
- → Parasympathetic nervous system
- → dorsal motor nucleus of the vagus nerve →
- → Hypothalamus (there: paraventricular nucleus) →
- Prelimbic cortex →
- → Nucleus ambiguus →
- → Parasympathetic nervous system
- → Nucleus ambiguus →
Source3
4. Stress reaction of the autonomic nervous system
4.1. Trigger
- Great effort
- Emotional stress
- Severe pain
- Severe fluid deficiency
4.2. Reaction
Noradrenaline activates other organs of the body via the sympathetic nervous system.
Adrenaline is released by the adrenal medulla.
- Increased heart rate
(noradrenaline and adrenaline via β1 receptors) - Accelerated breathing
(noradrenaline and adrenaline via β2 receptors) - Increase in blood pressure
(noradrenaline and adrenaline via alpha1 and β receptors) - Pupil dilation
- Increased supply of oxygen-rich blood to the skeletal muscles in preparation for the fight or flight response
- Noradrenaline and adrenaline reduce the blood supply to currently unimportant organs via β3 receptors
- Intestine
- Skin (reduce the risk of bleeding in the event of injury / combat, increase body heat)
- Stimulation of the liver to release energy-rich glucose
- Sweat glands activated (cold sweat)
- Stimulation of the adrenal gland
Strengthening the alert status by- Increased release of adrenaline
- Increased release of noradrenaline
4.3. Effect
- Increased alertness
- Increased flight behavior
- Increased energy consumption
A similar effect appears to exist in the central nervous system (brain and spinal cord), where the PFC is the “digestive” organ that is boosted by moderate levels of norepinephrine and shut down by high levels of norepinephrine, while the sensorimotor and affective regions of the brain are boosted by higher levels of norepinephrine.4
5. Alpha-amylase as a biomarker of the autonomic nervous system
Just as cortisol, the last hormone of the HPA axis, is a highly measurable biomarker of the HPA axis (e.g. in saliva), the alpha-amylase level reflects the reactivity of the sympathetic nervous system.56
Both biomarkers can be easily measured in saliva.
You can find out more about alpha-amylase in ADHD and its interaction with cortisol at ⇒ α-Amylase in ADHD And ⇒ Correlation between alpha-amylase and cortisol.
6. Measurement of the autonomic nervous system using heart rate variability (HRV)
The activity of the autonomic nervous system, in particular the parasympathetic nervous system, can be measured non-invasively by measuring heart rate variability. This results in interesting approaches to diagnostics and therapy.
⇒ Heart rate variability (HRV) in ADHD
7. Vegetative nervous system and ADHD
A meta-analysis of the autonomic nervous system in ADHD found7
- 2 studies reported a reduced sympathetic tone
- 7 studies reported lower sympathetic reactivity to task demands / stress
- 1 study reported reduced vagal tone
There was no evidence of altered task-related parasympathetic reactivity.
7.1. Adrenaline levels reduced in ADHD
Adrenaline is usually measured in the urine.
The basic functions of adrenaline: ⇒ neurotransmitters Neurotransmitters - messenger substances.
- A high adrenaline level correlates with faster decisions and fewer errors in cognitive tests in unstressed individuals, while a reduced adrenaline level correlates with slower decisions and higher error rates.8
- In a boring, under-stimulating task, (unstressed) subjects with higher adrenaline levels performed better than those with lower adrenaline levels. In contrast, subjects with lower adrenaline levels performed better in a demanding, overstimulating task.8
- Young men (average age 24) who showed a higher increase in noradrenaline and adrenaline in response to stress were more efficient in tests. This effect was even stronger for adrenaline than for noradrenaline.9
- Subjects whose adrenaline levels increased during an attention test compared to the waiting time achieved better results.10
- Children whose adrenaline levels increased during an arithmetic test compared to a passive situation performed better in the test than children who did not react with an adrenaline increase.11
- The subjective perception of stress correlates linearly with the level of adrenaline in healthy test subjects.12
- The adrenaline level (but not the noradrenaline level) in stressful situations tends to decrease with the feeling of control and manageability of the person with ADHD.13
- The adrenaline release of the sympathetic adrenal gland is significantly reduced in children with aggressiveness, motor restlessness and concentration difficulties under stress and without stress. Hyperactive boys show a significantly lower adrenaline release under stress as well as outside stress than those not affected. Low sympathetic-adrenal reactivity is discussed as a risk factor and susceptibility indicator for social and / or profound behavioral disorders.14
- People with depressive tendencies show a lower adrenaline stress response to acute stress than those who are not affected.15
7.2. Parasympathetic nervous system excessive and inflexible
One small study found in unmedicated children with ADHD:16
- Increased arousal of the parasympathetic nervous system
- Methylphenidate shifted the autonomic balance of children with ADHD towards normal values, but did not reach the comparative values of non-affected children
- MPH inhibits the normal response of the autonomic nervous system to a cognitive challenge.
- Methylphenidate appears to alter / suppress the normal stress response
Another study found differences in parasympathetic activity (PRS) in children with ADHD.17
Children with ADHD showed an inflexible, equal increase in PRS in each of
- Negative emotions
- Positive emotions
- Suppression of an activity
- Induction of an activity
Children without ADHD, on the other hand, showed
- Negative emotions: PRS increased more strongly
- Positive emotions: PRS less elevated
- Suppression of an activity: PRS more elevated
- Induction of an activity: PRS less elevated
A replication study confirmed the rigid pattern of increased PRS in children with ADHD, and also found an increased sympathetic nervous system response. The changes in the sympathetic nervous system in ADHD correlated with disorders of emotion reactivity, while the deviations in the parasympathetic nervous system correlated with disorders of emotion regulation.18
A study of children with and without ADHD found no striking differences in resting respiratory sinus arrhythmia (RSA) activity or reactivity. However, each correlated with the other regardless of ADHD status:19
- Reduced prosocial behavior with
- Lower RSA value at rest
- Lower reactive RSA decline
- Emotion regulation problems with
- Increased reactive RSA decline to incentives.
Respiratory sinus arrhythmia (RSA) consists of oscillatory increases and decreases in heart rate during the respiratory cycle. It represents parasympathetic / vagal effects on the heart. The RSA is thought to represent neuronal traffic through the vagus nerve20. The vagus nerve is thought to represent a physiological mechanism for the rapid acceleration and deceleration of cardiac output in response to environmental (including social) demands.21
7.3. Inconsistent findings on the sympathetic nervous system in ADHD
The cardiac pre-ejection period (PEP) is a systolic time interval mediated by the sympathetic nervous system (SNS) that encompasses the depolarization of the left ventricle until the start of blood ejection into the aorta (the time from the start of electrical stimulation of the left ventricle (start of the Q-wave in the ECG) until the opening of the aortic valve). PEP represents mesolimbic dopamine reactivity, particularly during the reward response.22 A longer PEP is a marker for reduced activity of the sympathetic nervous system, although this can also be influenced by other factors.23
7.3.1. Attenuated sympathetic nervous system: 2 studies
A study of 2,209 participants found a correlation between inattention and a prolonged pre-ejection period (PEP), indicating a weakened sympathetic nervous system in relation to inattention.24 A small study also found an underarousal of the sympathetic nervous system in unmedicated children with ADHD16
7.3.2. Sympathetic nervous system unchanged: 2 studies
One study found no abnormalities of the sympathetic nervous system in ADHD17
A study of children with and without ADHD found no resounding differences in resting activity or cardiac pre-ejection period (PEP) reactivity. However, each correlated with the other regardless of ADHD status:19
- Behavioral problems and aggression with
- Extended PEP at rest
- Reduced PEP reactivity to incentives
Sympathetic nervous system is increased:
In contrast, another study found an increased sympathetic nervous system response in children with ADHD. The changes in the sympathetic nervous system in ADHD correlated with disorders of emotion reactivity, while the deviations in the parasympathetic nervous system correlated with disorders of emotion regulation.18
7.3.3. Sympathetic nervous system increased: 2 studies
In ADHD, HRV is reduced and the sympathetic nervous system is overactivated.25 Together with reduced cardiac-linked parasympathetic activity and reduced HRV, this is a non-invasive marker for prefrontal hypoactivity and ADHD.26
Further studies found evidence of an increased sympathetic nervous system in comorbid externalizing disorders
One study found a significant reduction in electrodermal activity in adolescents with ADHD with and without comorbid conduct disorder, which is consistent with lower anxiety levels in impulsivity. An attenuated PEP response to reward was only found in adolescents with ADHD and comorbid Conduct Disorder, not ADHD alone.27 Further studies also suggest that reduced reward reactivity of the mesolimbic dopaminergic system is reflected in attenuated PEP signals to reward and correlates particularly with aggressive externalizing behaviour 28 2930
Two studies that found decreased heart rate variability on rewards in ADHD did not differentiate between ADHD and comorbid externalizing disorders.3132
8. Changes in the autonomic nervous system due to ADHD medication
A study on adolescents with ADHD found reduced activity of the sympathetic and parasympathetic nervous system compared to those not affected. This difference was almost equalized by a sustained release MPH preparation.33
9. Parasympathetic / sympathetic nervous system for other disorders
Borderline personality disorder showed increased sympathetic tone with and without comorbid ADHD, as evidenced by larger pupils than in control subjects.34
Bellato, Arora, Hollis, Groom (2019): Is autonomic nervous system function atypical in Attention Deficit Hyperactivity Disorder (ADHD)? A systematic review of the evidence. Neurosci Biobehav Rev. 2019 Nov 10. pii: S0149-7634(19)30418-X. doi: 10.1016/j.neubiorev.2019.11.001. ↥
Wolf, Calabrese (2020): Stressmedizin & Stresspsychologie, S. 73 ↥ ↥
Ulrich-Lai, Herman (2009): Neural Regulation of Endocrine and Autonomic Stress Responses; Nat Rev Neurosci. 2009 Jun; 10(6): 397–409.; doi: 10.1038/nrn2647 ↥ ↥
Ramos, Arnsten (2007): Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007 Mar; 113(3):523-36., Kapitel 6 ↥
Nater, Rohleder (2009): Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 2009;34(4):486–96. ↥
Nater, Rohleder, Gaab, Berger, Jud (2005): Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol 2005;55(3):333–42. ↥
Geiss L, Stemmler M, Beck B, Hillemacher T, Widder M, Hösl KM. Dysregulation of the autonomic nervous system in adult attention deficit hyperactivity disorder. A systematic review. Cogn Neuropsychiatry. 2023 Sep 13:1-22. doi: 10.1080/13546805.2023.2255336. PMID: 37702351. METASTUDY, k = 15, n = 846 ↥
Frankenhaeuser (1971): Behavior and circulating catecholamines. Brain Research, 31(2), 241-262. http://dx.doi.org/10.1016/0006-8993(71)90180-6 ↥ ↥
Frankenhaeuser, Mellis, Rissler, Bjorkvall, Patkai (1968): Catecholamine excretion as related to cognitive and emotional reaction patterns, Psychosom, Med., 30 (1968) 109-120., n = 25 ↥
Frankenhaeuser, Nordheden, Myrsten, Post (1970): Psychophysiological reactions to understimulation and overstimulation, Department of Psychology Research Report, 36. Stockholm: University of Stockholm, (1970) No. 316., zitiert nach Frankenhaeuser (1971): Behavior and circulating catecholamines. Brain Research, 31(2), 241-262. http://dx.doi.org/10.1016/0006-8993(71)90180-6, Seite 252 ↥
Johanssson (1970): Katekolaminutsiöndring och beteende hos barn, (Catecholamine release and behavior in children), unpublished thesis, Univ. Stockholm, (1970), zitiert nach Frankenhaeuser (1971): Behavior and circulating catecholamines. Brain Research, 31(2), 241-262. http://dx.doi.org/10.1016/0006-8993(71)90180-6, Seite 252 ↥
Frankenhaeuser, Sterky, Jarpe (1962): Psychophysiological relations in habituation to gravitational stress, Percept. mot. Skills, 15 (1962) 63-72. ↥
Frankenhaeuser, Rissler (1970): Effects of punishment on catecholamine release and efficiency of performance, Psychopharmacologia (Berl.), 17 (1970) 378-390. ↥
Klinteberg, Magnussen (1989): Aggressiveness and hyperactive behaviour as related to adrenaline excretion. Europ J Personality 3: 81-93 ↥
Frankenhaeuser, Patkai (1965): lnterindividual differences in catecholamine excretion during stress, Scand. J. Psychol., 6 (1965) 117-123. n = 110 ↥
Negrao, Bipath, van der Westhuizen, Viljoen (2011): Autonomic correlates at rest and during evoked attention in children with attention-deficit/hyperactivity disorder and effects of methylphenidate. Neuropsychobiology. 2011;63(2):82-91. doi: 10.1159/000317548. PMID: 21178382. n = 37 ↥ ↥
Musser, Backs, Schmitt, Ablow, Measelle, Nigg (2011, 2018): Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD). J Abnorm Child Psychol. 2011 Aug;39(6):841-52. doi: 10.1007/s10802-011-9499-1. Erratum in: J Abnorm Child Psychol. 2018 Jan 26;: PMID: 21394506; PMCID: PMC3112468. n = 66 ↥ ↥
Morris, Musser, Tenenbaum, Ward, Martinez, Raiker, Coles, Riopelle (2020): Emotion Regulation via the Autonomic Nervous System in Children with Attention-Deficit/Hyperactivity Disorder (ADHD): Replication and Extension. J Abnorm Child Psychol. 2020 Mar;48(3):361-373. doi: 10.1007/s10802-019-00593-8. PMID: 31808007; PMCID: PMC7720673. n = 259 ↥ ↥
Beauchaine, Gatzke-Kopp, Neuhaus, Chipman, Reid, Webster-Stratton (2013): Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. J Consult Clin Psychol. 2013 Jun;81(3):481-493. doi: 10.1037/a0032302. PMID: 23544677; PMCID: PMC3952490. n = 99 ↥ ↥
[Ritz (2009): Studying noninvasive indices of vagal control: the need for respiratory control and the problem of target specificity. Biol Psychol. 2009 Feb;80(2):158-68. doi: 10.1016/j.biopsycho.2008.08.003. PMID: 18775468.](https://pubmed.ncbi.nlm.nih.gov/18775468/ ↥
Porges (2007): The polyvagal perspective. Biol Psychol. 2007 Feb;74(2):116-43. doi: 10.1016/j.biopsycho.2006.06.009. PMID: 17049418; PMCID: PMC1868418. REVIEW ↥
Brenner, Beauchaine (2011): Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: a pilot study. Psychophysiology. 2011 Nov;48(11):1588-1596. doi: 10.1111/j.1469-8986.2011.01230.x. PMID: 21729103. ↥
Krohova, Czippelova, Turianikova, Lazarova, Tonhajzerova, Javorka (2017): Preejection period as a sympathetic activity index: a role of confounding factors. Physiol Res. 2017 Sep 22;66(Supplementum 2):S265-S275. ↥
Vogel, Bijlenga, Verduijn, Bron, Beekman, Kooij, Penninx (2017): Attention-deficit/hyperactivity disorder symptoms and stress-related biomarkers. Psychoneuroendocrinology. 2017 May;79:31-39. doi: 10.1016/j.psyneuen.2017.02.009. n = 2.209 ↥
Tonhajzerova, Farsky, Mestanik, Visnovcova, Mestanikova, Hrtanek, Ondrejka (2016): Symbolic dynamics of heart rate variability – a promising tool to investigate cardiac sympathovagal control in attention deficit/hyperactivity disorder (ADHD)? Can J Physiol Pharmacol. 2016 Jun;94(6):579-87. doi: 10.1139/cjpp-2015-0375. ↥
Sekaninova, Mestanik, Mestanikova, Hamrakova, Tonhajzerova (2019): Novel approach to evaluate central autonomic regulation in attention deficit/hyperactivity disorder (ADHD). Physiol Res. 2019 Aug 29;68(4):531-545. ↥
Beauchaine, Katkin, Strassberg, Snarr (2001): Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. J Abnorm Psychol. 2001 Nov;110(4):610-24. doi: 10.1037//0021-843x.110.4.610. PMID: 11727950. ↥
Gatzke-Kopp, Beauchaine (2007): Central nervous system substrates of impulsivity: Implications for the development of attention-deficit/hyperactivity disorder and conduct disorder. In: Coch, Dawson, Fischer ( Eds): Human behavior, learning, and the developing brain: Atypical development. New York: Guilford Press; 2007. pp. 239–263; 247 ↥
Beauchaine, Gatzke-Kopp, Mead (2007): Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007 Feb;74(2):174-84. doi: 10.1016/j.biopsycho.2005.08.008. PMID: 17045726; PMCID: PMC1801075. REVIEW ↥
Crowell, Beauchaine, Gatzke-Kopp, Sylvers, Mead, Chipman-Chacon (2006): Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. J Abnorm Psychol. 2006 Feb;115(1):174-8. doi: 10.1037/0021-843X.115.1.174. PMID: 16492108. ↥
Crone, Jennings, van der Molen (2003): Sensitivity to interference and response contingencies in attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2003 Feb;44(2):214-26. doi: 10.1111/1469-7610.00115. PMID: 12587858. ↥
Iaboni, Douglas, Ditto (1997): Psychophysiological response of ADHD children to reward and extinction. Psychophysiology. 1997 Jan;34(1):116-23. doi: 10.1111/j.1469-8986.1997.tb02422.x. PMID: 9009815. ↥
Morris, Musser, Tenenbaum, Ward, Raiker, Coles (2021): Methylphenidate Improves Autonomic Functioning among Youth with Attention-Deficit/Hyperactivity Disorder. Res Child Adolesc Psychopathol. 2021 Oct 6. doi: 10.1007/s10802-021-00870-5. PMID: 34613513. ↥
Calancie OG, Parr AC, Brien DC, Huang J, Pitigoi IC, Coe BC, Booij L, Khalid-Khan S, Munoz DP (2023): Motor synchronization and impulsivity in pediatric borderline personality disorder with and without attention-deficit hyperactivity disorder: an eye-tracking study of saccade, blink and pupil behavior. Front Neurosci. 2023 Jun 22;17:1179765. doi: 10.3389/fnins.2023.1179765. PMID: 37425020; PMCID: PMC10323365. ↥
